Energy Storage Materials ( IF 18.9 ) Pub Date : 2020-02-05 , DOI: 10.1016/j.ensm.2020.01.032 Zhao Cai , Yangtao Ou , Jindi Wang , Run Xiao , Lin Fu , Zhu Yuan , Renmin Zhan , Yongming Sun
|
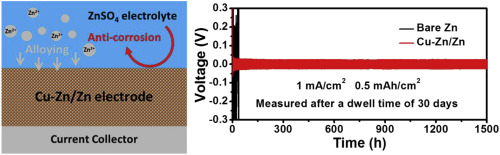
Rechargeable aqueous Zn metal batteries are promising candidates for renewable energy storage. However, Zn metal is chemically active and suffers from chemical corrosion in aqueous electrolyte due to its low redox potential. It is of vital importance to reveal the corrosion mechanism, and improve the chemical stability and electrochemical reversibility of Zn metal anode for its practical application. In this work, it is revealed that a Zn metal electrode readily gets oxidized during its resting in aqueous ZnSO4 electrolyte, forming zinc hydroxide sulfate and hydrogen gas, leading to the increased internal resistance and swollen problems of batteries, and eventually battery failure. To inhibit such chemical corrosion, an anti-corrosive metallic Cu is introduced to Zn metal anode to construct a uniform Cu/Zn composite with dense structure, which is electrochemically converted to Cu-Zn alloy/Zn composite during battery cycling. The as-achieved Cu-Zn/Zn electrode exhibits stable cycling for over 1500 cycles at 1 mA/cm2 and 0.5 mAh/cm2 with little change in overpotential (46 mV) after resting for 1 month, while the bare Zn electrode shows large voltage fluctuation and high overpotential (>400 mV) under the same condition, suggesting the importance of inhibiting the chemical corrosion of Zn metal anode for rechargeable aqueous batteries.
中文翻译:

长周期水性电池用耐化学腐蚀的Cu-Zn / Zn复合阳极
可充电含水锌金属电池是可再生能源存储的有前途的候选者。然而,Zn金属具有化学活性,并且由于其低氧化还原电势而在水性电解质中遭受化学腐蚀。揭示其腐蚀机理,提高锌金属阳极的化学稳定性和电化学可逆性对其实际应用至关重要。在这项工作中,揭示了锌金属电极在置于ZnSO 4水溶液中时容易被氧化。电解液,形成氢氧化锌硫酸盐和氢气,导致内部电阻增加和电池溶胀问题,最终导致电池故障。为了抑制这种化学腐蚀,将防腐金属Cu引入到Zn金属阳极中,以构造具有致密结构的均匀Cu / Zn复合材料,在电池循环过程中将其电化学转化为Cu-Zn合金/ Zn复合材料。所获得的Cu-Zn / Zn电极在1 mA / cm 2和0.5 mAh / cm 2的条件下在超过1500个循环中表现出稳定的循环 静置1个月后的超电势(46 mV)几乎没有变化,而裸露的Zn电极在相同条件下显示出大的电压波动和高的超电势(> 400 mV),这表明了抑制Zn金属阳极化学腐蚀的重要性。可充电水性电池。











































 京公网安备 11010802027423号
京公网安备 11010802027423号