当前位置:
X-MOL 学术
›
Desalination
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Eutectic freeze crystallization of saline dairy effluent
Desalination ( IF 9.9 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.desal.2020.114349 G.Q. Chen , S.L. Gras , S.E. Kentish
Desalination ( IF 9.9 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.desal.2020.114349 G.Q. Chen , S.L. Gras , S.E. Kentish
|
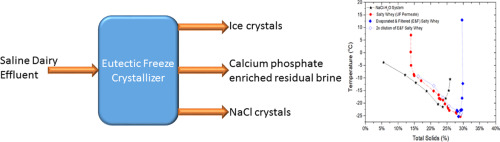
Abstract The disposal of saline effluent in the dairy industry is subject to increasingly strict regulatory requirements. In this work, eutectic freeze crystallization (EFC) was investigated as a mechanism for the simultaneous separation of salts and ice in a typical saline effluent, namely salty whey. Experiments were conducted using salty whey samples collected from a dairy processing facility. The eutectic point of the salty whey was determined using differential scanning calorimetry and was found to be lower than that of NaCl solutions (−24 °C for salty whey vs. −21 °C for aqueous NaCl solutions). Crystallization experiments were then used to construct the phase diagram of this dairy stream under equilibrium conditions. The change in cation composition in the supernatant at the eutectic temperature was measured as a function of time and showed that pure NaCl salts and ice formed within 5 min after this temperature was reached. The energy consumption of this process was estimated to be ~120 kWh/t for salty whey, which is comparable to that for conventional thermal crystallization of brine.
中文翻译:

含盐乳品废水的共晶冷冻结晶
摘要 乳品行业含盐废水的处理受到越来越严格的监管要求。在这项工作中,共晶冷冻结晶 (EFC) 被研究为一种同时分离典型盐水流出物(即咸乳清)中盐和冰的机制。使用从乳制品加工设施收集的咸乳清样品进行实验。使用差示扫描量热法测定咸乳清的共晶点,发现其低于 NaCl 溶液(咸乳清为 -24 °C,而 NaCl 水溶液为 -21 °C)。然后使用结晶实验来构建该乳制品流在平衡条件下的相图。在共晶温度下上清液中阳离子组成的变化作为时间的函数进行测量,表明在达到该温度后 5 分钟内形成了纯 NaCl 盐和冰。该过程的能耗估计约为 120 kWh/t 咸乳清,与传统的盐水热结晶相当。
更新日期:2020-04-01
中文翻译:

含盐乳品废水的共晶冷冻结晶
摘要 乳品行业含盐废水的处理受到越来越严格的监管要求。在这项工作中,共晶冷冻结晶 (EFC) 被研究为一种同时分离典型盐水流出物(即咸乳清)中盐和冰的机制。使用从乳制品加工设施收集的咸乳清样品进行实验。使用差示扫描量热法测定咸乳清的共晶点,发现其低于 NaCl 溶液(咸乳清为 -24 °C,而 NaCl 水溶液为 -21 °C)。然后使用结晶实验来构建该乳制品流在平衡条件下的相图。在共晶温度下上清液中阳离子组成的变化作为时间的函数进行测量,表明在达到该温度后 5 分钟内形成了纯 NaCl 盐和冰。该过程的能耗估计约为 120 kWh/t 咸乳清,与传统的盐水热结晶相当。



























 京公网安备 11010802027423号
京公网安备 11010802027423号