当前位置:
X-MOL 学术
›
Transl. Psychiaty
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comprehensive analysis of a novel mouse model of the 22q11.2 deletion syndrome: a model with the most common 3.0-Mb deletion at the human 22q11.2 locus.
Translational Psychiatry ( IF 5.8 ) Pub Date : 2020-02-05 , DOI: 10.1038/s41398-020-0723-z Ryo Saito 1, 2 , Michinori Koebis 1 , Taku Nagai 3 , Kimiko Shimizu 2 , Jingzhu Liao 3 , Bolati Wulaer 3 , Yuki Sugaya 4, 5 , Kenichiro Nagahama 4, 5 , Naofumi Uesaka 4, 5 , Itaru Kushima 6, 7 , Daisuke Mori 6 , Kazuaki Maruyama 8 , Kazuki Nakao 1 , Hiroki Kurihara 8 , Kiyofumi Yamada 3 , Masanobu Kano 4, 5 , Yoshitaka Fukada 2 , Norio Ozaki 6 , Atsu Aiba 1, 2
Translational Psychiatry ( IF 5.8 ) Pub Date : 2020-02-05 , DOI: 10.1038/s41398-020-0723-z Ryo Saito 1, 2 , Michinori Koebis 1 , Taku Nagai 3 , Kimiko Shimizu 2 , Jingzhu Liao 3 , Bolati Wulaer 3 , Yuki Sugaya 4, 5 , Kenichiro Nagahama 4, 5 , Naofumi Uesaka 4, 5 , Itaru Kushima 6, 7 , Daisuke Mori 6 , Kazuaki Maruyama 8 , Kazuki Nakao 1 , Hiroki Kurihara 8 , Kiyofumi Yamada 3 , Masanobu Kano 4, 5 , Yoshitaka Fukada 2 , Norio Ozaki 6 , Atsu Aiba 1, 2
Affiliation
|
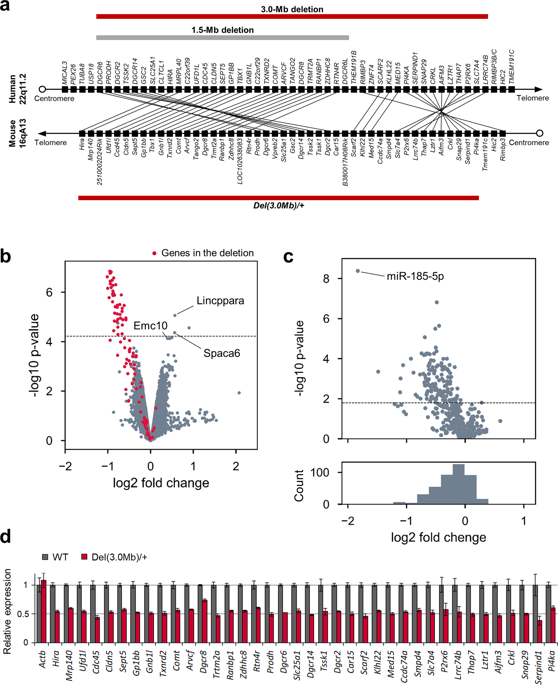
The 22q11.2 deletion syndrome (22q11.2DS) is associated with an increased risk for psychiatric disorders. Although most of the 22q11.2DS patients have a 3.0-Mb deletion, existing mouse models only mimic a minor mutation of 22q11.2DS, a 1.5-Mb deletion. The role of the genes existing outside the 1.5-Mb deletion in psychiatric symptoms of 22q11.2DS is unclear. In this study, we generated a mouse model that reproduced the 3.0-Mb deletion of the 22q11.2DS (Del(3.0 Mb)/ +) using the CRISPR/Cas9 system. Ethological and physiological phenotypes of adult male mutants were comprehensively evaluated by visual-evoked potentials, circadian behavioral rhythm, and a series of behavioral tests, such as measurement of locomotor activity, prepulse inhibition, fear-conditioning memory, and visual discrimination learning. As a result, Del(3.0 Mb)/ + mice showed reduction of auditory prepulse inhibition and attenuated cue-dependent fear memory, which is consistent with the phenotypes of existing 22q11.2DS models. In addition, Del(3.0 Mb)/ + mice displayed an impaired early visual processing that is commonly seen in patients with schizophrenia. Meanwhile, unlike the existing models, Del(3.0 Mb)/ + mice exhibited hypoactivity over several behavioral tests, possibly reflecting the fatigability of 22q11.2DS patients. Lastly, Del(3.0 Mb)/ + mice displayed a faster adaptation to experimental jet lag as compared with wild-type mice. Our results support the validity of Del(3.0 Mb)/ + mice as a schizophrenia animal model and suggest that our mouse model is a useful resource to understand pathogenic mechanisms of schizophrenia and other psychiatric disorders associated with 22q11.2DS.
中文翻译:

22q11.2 缺失综合征新型小鼠模型的综合分析:人类 22q11.2 基因座最常见的 3.0-Mb 缺失模型。
22q11.2 缺失综合征 (22q11.2DS) 与精神疾病风险增加有关。尽管大多数 22q11.2DS 患者有 3.0-Mb 缺失,但现有的小鼠模型仅模拟 22q11.2DS 的微小突变,即 1.5-Mb 缺失。存在于 1.5-Mb 缺失之外的基因在 22q11.2DS 的精神症状中的作用尚不清楚。在这项研究中,我们使用 CRISPR/Cas9 系统生成了一个小鼠模型,该模型再现了 22q11.2DS (Del(3.0 Mb)/ +) 的 3.0-Mb 缺失。通过视觉诱发电位、昼夜行为节律和一系列行为测试,如运动活动测量、前脉冲抑制、恐惧条件记忆和视觉辨别学习等,对成年男性突变体的行为学和生理表型进行了综合评估。因此,德尔(3。0 Mb)/ + 小鼠表现出听觉前脉冲抑制减少和线索依赖性恐惧记忆减弱,这与现有 22q11.2DS 模型的表型一致。此外,Del(3.0 Mb)/ + 小鼠表现出早期视觉处理受损,这在精神分裂症患者中很常见。同时,与现有模型不同,Del(3.0 Mb)/ + 小鼠在多次行为测试中表现出低活性,可能反映了 22q11.2DS 患者的易疲劳性。最后,与野生型小鼠相比,Del(3.0 Mb)/ + 小鼠对实验时差的适应更快。我们的结果支持 Del(3.0 Mb)/ + 小鼠作为精神分裂症动物模型的有效性,并表明我们的小鼠模型是了解精神分裂症和其他与 22q11.2DS 相关的精神疾病的发病机制的有用资源。
更新日期:2020-02-06
中文翻译:

22q11.2 缺失综合征新型小鼠模型的综合分析:人类 22q11.2 基因座最常见的 3.0-Mb 缺失模型。
22q11.2 缺失综合征 (22q11.2DS) 与精神疾病风险增加有关。尽管大多数 22q11.2DS 患者有 3.0-Mb 缺失,但现有的小鼠模型仅模拟 22q11.2DS 的微小突变,即 1.5-Mb 缺失。存在于 1.5-Mb 缺失之外的基因在 22q11.2DS 的精神症状中的作用尚不清楚。在这项研究中,我们使用 CRISPR/Cas9 系统生成了一个小鼠模型,该模型再现了 22q11.2DS (Del(3.0 Mb)/ +) 的 3.0-Mb 缺失。通过视觉诱发电位、昼夜行为节律和一系列行为测试,如运动活动测量、前脉冲抑制、恐惧条件记忆和视觉辨别学习等,对成年男性突变体的行为学和生理表型进行了综合评估。因此,德尔(3。0 Mb)/ + 小鼠表现出听觉前脉冲抑制减少和线索依赖性恐惧记忆减弱,这与现有 22q11.2DS 模型的表型一致。此外,Del(3.0 Mb)/ + 小鼠表现出早期视觉处理受损,这在精神分裂症患者中很常见。同时,与现有模型不同,Del(3.0 Mb)/ + 小鼠在多次行为测试中表现出低活性,可能反映了 22q11.2DS 患者的易疲劳性。最后,与野生型小鼠相比,Del(3.0 Mb)/ + 小鼠对实验时差的适应更快。我们的结果支持 Del(3.0 Mb)/ + 小鼠作为精神分裂症动物模型的有效性,并表明我们的小鼠模型是了解精神分裂症和其他与 22q11.2DS 相关的精神疾病的发病机制的有用资源。










































 京公网安备 11010802027423号
京公网安备 11010802027423号