当前位置:
X-MOL 学术
›
Regul. Toxicol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preclinical pharmacokinetic, biodistribution, radiation dosimetry, and toxicity studies of 99mTc-HYNIC-(Ser)3-LTVPWY: A novel HER2-targeted peptide radiotracer.
Regulatory Toxicology and Pharmacology ( IF 3.0 ) Pub Date : 2020-02-05 , DOI: 10.1016/j.yrtph.2020.104591 Javad Biabani Ardakani 1 , Fereshteh Talebpour Amiri 2 , Alireza Khorramimoghaddam 3 , Ali Abbasi 4 , Sajjad Molavipordanjani 5 , Seyed Jalal Hosseinimehr 5
Regulatory Toxicology and Pharmacology ( IF 3.0 ) Pub Date : 2020-02-05 , DOI: 10.1016/j.yrtph.2020.104591 Javad Biabani Ardakani 1 , Fereshteh Talebpour Amiri 2 , Alireza Khorramimoghaddam 3 , Ali Abbasi 4 , Sajjad Molavipordanjani 5 , Seyed Jalal Hosseinimehr 5
Affiliation
|
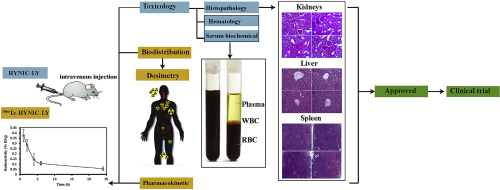
Accurate assessment of the HER2 expression is an essential issue for predicting response to anti-HER2 therapy in breast cancer patients. The aim of this study was to evaluate 99mTc-HYNIC-(Ser)3-LTVPWY (99mTc-HYNIC-LY) peptide as a novel HER2-targeted radiolabeled peptide in healthy mice to examine the applicability of this imaging agent in a first-in-human clinical trial. To this end, pharmacokinetic and dosimetry studies were performed according to the ICH guideline M3 (R2) with 99mTc-HYNIC-LY. To estimate the radiation-absorbed doses in humans, the accumulated activity in each mouse organ was calculated based on biodistribution data. In addition, toxicology assessment was performed based on mortality events, body weights, and serum biochemical, hematological, and histopathological assays. The pharmacokinetic study showed rapid blood clearance. Based on the results of biodistribution study, the highest radioactivity was observed in the kidneys. The projected absorbed doses to the kidneys, liver, lungs, stomach, and spleen were obtained as 1.70E-02, 1.42E-02, 1.02E-02, 8.62E-03, and 8.34E-03 mSv/MBq, respectively. The results also revealed that serum biochemical and hematological parameters were in the normal range. No significant morphologic alterations were observed in the liver, kidneys, and spleen tissues. Consequently, the results were indicative of the suitability of 99mTc-HYNIC-LY peptide for advancement to a first-in-human clinical trial.
中文翻译:

99mTc-HYNIC-(Ser)3-LTVPWY的临床前药代动力学,生物分布,辐射剂量测定和毒性研究:一种靶向HER2的新型肽放射性示踪剂。
HER2表达的准确评估对于预测乳腺癌患者对抗HER2治疗的反应至关重要。这项研究的目的是评估99mTc-HYNIC-(Ser)3-LTVPWY(99mTc-HYNIC-LY)肽作为健康小鼠中靶向HER2的新型肽,以检查该显像剂在首创中的适用性-人体临床试验。为此,根据ICH指南M3(R2)和99mTc-HYNIC-LY进行了药代动力学和剂量学研究。为了估计人体中的辐射吸收剂量,根据生物分布数据计算了每个小鼠器官中的累积活性。此外,根据死亡事件,体重以及血清生化,血液学和组织病理学分析进行了毒理学评估。药代动力学研究表明血液清除迅速。根据生物分布研究的结果,在肾脏中观察到最高的放射性。获得的对肾脏,肝,肺,胃和脾的预计吸收剂量分别为1.70E-02、1.42E-02、1.02E-02、8.62E-03和8.34E-03 mSv / MBq。结果还显示血清生化和血液学参数在正常范围内。在肝,肾和脾组织中未观察到明显的形态学改变。因此,该结果表明99mTc-HYNIC-LY肽适用于人类首次临床试验。分别为34E-03 mSv / MBq。结果还显示血清生化和血液学参数在正常范围内。在肝,肾和脾组织中未观察到明显的形态学改变。因此,该结果表明99mTc-HYNIC-LY肽适用于人类首次临床试验。分别为34E-03 mSv / MBq。结果还显示血清生化和血液学参数在正常范围内。在肝,肾和脾组织中未观察到明显的形态学改变。因此,该结果表明99mTc-HYNIC-LY肽适用于人类首次临床试验。
更新日期:2020-02-06
中文翻译:

99mTc-HYNIC-(Ser)3-LTVPWY的临床前药代动力学,生物分布,辐射剂量测定和毒性研究:一种靶向HER2的新型肽放射性示踪剂。
HER2表达的准确评估对于预测乳腺癌患者对抗HER2治疗的反应至关重要。这项研究的目的是评估99mTc-HYNIC-(Ser)3-LTVPWY(99mTc-HYNIC-LY)肽作为健康小鼠中靶向HER2的新型肽,以检查该显像剂在首创中的适用性-人体临床试验。为此,根据ICH指南M3(R2)和99mTc-HYNIC-LY进行了药代动力学和剂量学研究。为了估计人体中的辐射吸收剂量,根据生物分布数据计算了每个小鼠器官中的累积活性。此外,根据死亡事件,体重以及血清生化,血液学和组织病理学分析进行了毒理学评估。药代动力学研究表明血液清除迅速。根据生物分布研究的结果,在肾脏中观察到最高的放射性。获得的对肾脏,肝,肺,胃和脾的预计吸收剂量分别为1.70E-02、1.42E-02、1.02E-02、8.62E-03和8.34E-03 mSv / MBq。结果还显示血清生化和血液学参数在正常范围内。在肝,肾和脾组织中未观察到明显的形态学改变。因此,该结果表明99mTc-HYNIC-LY肽适用于人类首次临床试验。分别为34E-03 mSv / MBq。结果还显示血清生化和血液学参数在正常范围内。在肝,肾和脾组织中未观察到明显的形态学改变。因此,该结果表明99mTc-HYNIC-LY肽适用于人类首次临床试验。分别为34E-03 mSv / MBq。结果还显示血清生化和血液学参数在正常范围内。在肝,肾和脾组织中未观察到明显的形态学改变。因此,该结果表明99mTc-HYNIC-LY肽适用于人类首次临床试验。










































 京公网安备 11010802027423号
京公网安备 11010802027423号