当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological activity of N5-substituted tetrahydropteroate analogs as non-classical antifolates against cobalamin-dependent methionine synthase and potential anticancer agents.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-05 , DOI: 10.1016/j.ejmech.2020.112113 Meng Wang 1 , Chao Tian 2 , Liangmin Xue 2 , Hao Li 2 , Jing Cong 2 , Fang Fang 2 , Jiajia Yang 2 , Mengmeng Yuan 2 , Ying Chen 2 , Ying Guo 2 , Xiaowei Wang 2 , Junyi Liu 3 , Zhili Zhang 2
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-05 , DOI: 10.1016/j.ejmech.2020.112113 Meng Wang 1 , Chao Tian 2 , Liangmin Xue 2 , Hao Li 2 , Jing Cong 2 , Fang Fang 2 , Jiajia Yang 2 , Mengmeng Yuan 2 , Ying Chen 2 , Ying Guo 2 , Xiaowei Wang 2 , Junyi Liu 3 , Zhili Zhang 2
Affiliation
|
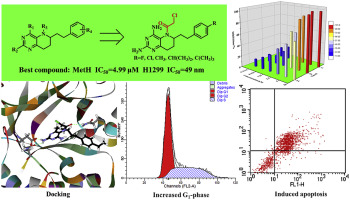
Cobalamin-dependent methionine synthase (MetH) is involved in the process of tumor cell growth and survival. In this study, a novel series of N5-electrophilic substituted tetrahydropteroate analogs without glutamate residue were designed as non-classical antifolates and evaluated for their inhibitory activities against MetH. In addition, the cytotoxicity of target compounds was evaluated in human tumor cell lines. With N5-chloracetyl as the optimum group, further structure research on the benzene substituent and on the 2,4-diamino group was also performed. Compound 6c, with IC50 value of 12.1 μM against MetH and 0.16-6.12 μM against five cancer cells, acted as competitive inhibitor of MetH. Flow cytometry studies indicated that compound 6c arrested HL-60 cells in the G1-phase and then inducted late apoptosis. The molecular docking further explained the structure-activity relationship.
中文翻译:

N5取代的四氢蝶呤类似物作为非经典抗叶酸剂的设计,合成和生物学活性,可抵抗钴胺素依赖性蛋氨酸合酶和潜在的抗癌剂。
钴胺素依赖性蛋氨酸合酶(MetH)参与肿瘤细胞的生长和存活过程。在这项研究中,没有谷氨酸残基的一系列新的N5-亲电子取代的四氢蝶呤类似物被设计为非经典的抗叶酸药物,并评估了它们对MetH的抑制活性。另外,在人肿瘤细胞系中评估了目标化合物的细胞毒性。以N5-氯乙酰基为最佳基团,还对苯取代基和2,4-二氨基进行了进一步的结构研究。化合物6c,作为MetH的竞争性抑制剂,对MetH的IC50值为12.1μM,对五个癌细胞的IC50值为0.16-6.12μM。流式细胞术研究表明化合物6c将HL-60细胞阻滞在G1期,然后诱导晚期凋亡。
更新日期:2020-02-06
中文翻译:

N5取代的四氢蝶呤类似物作为非经典抗叶酸剂的设计,合成和生物学活性,可抵抗钴胺素依赖性蛋氨酸合酶和潜在的抗癌剂。
钴胺素依赖性蛋氨酸合酶(MetH)参与肿瘤细胞的生长和存活过程。在这项研究中,没有谷氨酸残基的一系列新的N5-亲电子取代的四氢蝶呤类似物被设计为非经典的抗叶酸药物,并评估了它们对MetH的抑制活性。另外,在人肿瘤细胞系中评估了目标化合物的细胞毒性。以N5-氯乙酰基为最佳基团,还对苯取代基和2,4-二氨基进行了进一步的结构研究。化合物6c,作为MetH的竞争性抑制剂,对MetH的IC50值为12.1μM,对五个癌细胞的IC50值为0.16-6.12μM。流式细胞术研究表明化合物6c将HL-60细胞阻滞在G1期,然后诱导晚期凋亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号