当前位置:
X-MOL 学术
›
Gastroenterology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
GPR30-Expressing Gastric Chief Cells Do Not Dedifferentiate But Are Eliminated via PDK-Dependent Cell Competition During Development of Metaplasia.
Gastroenterology ( IF 25.7 ) Pub Date : 2020-02-04 , DOI: 10.1053/j.gastro.2020.01.046 Masahiro Hata 1 , Hiroto Kinoshita 2 , Yoku Hayakawa 1 , Mitsuru Konishi 1 , Mayo Tsuboi 1 , Yukiko Oya 1 , Ken Kurokawa 1 , Yuki Hayata 1 , Hayato Nakagawa 1 , Keisuke Tateishi 1 , Hiroaki Fujiwara 3 , Yoshihiro Hirata 4 , Daniel L Worthley 5 , Yuki Muranishi 6 , Takahisa Furukawa 6 , Shunsuke Kon 7 , Hiroyuki Tomita 8 , Timothy C Wang 9 , Kazuhiko Koike 1
Gastroenterology ( IF 25.7 ) Pub Date : 2020-02-04 , DOI: 10.1053/j.gastro.2020.01.046 Masahiro Hata 1 , Hiroto Kinoshita 2 , Yoku Hayakawa 1 , Mitsuru Konishi 1 , Mayo Tsuboi 1 , Yukiko Oya 1 , Ken Kurokawa 1 , Yuki Hayata 1 , Hayato Nakagawa 1 , Keisuke Tateishi 1 , Hiroaki Fujiwara 3 , Yoshihiro Hirata 4 , Daniel L Worthley 5 , Yuki Muranishi 6 , Takahisa Furukawa 6 , Shunsuke Kon 7 , Hiroyuki Tomita 8 , Timothy C Wang 9 , Kazuhiko Koike 1
Affiliation
|
BACKGROUND & AIMS
Gastric chief cells, a mature cell type that secretes digestive enzymes, have been proposed to be the origin of metaplasia and cancer through dedifferentiation or transdifferentiation. However, studies supporting this claim have had technical limitations, including issues with the specificity of chief cell markers and the toxicity of drugs used. We therefore sought to identify genes expressed specifically in chief cells and establish a model to trace these cells.
METHODS
We performed transcriptome analysis of Mist1-CreERT-traced cells, with or without chief cell depletion. Gpr30-rtTA mice were generated and crossed to TetO-Cre mice, and lineage tracing was performed after crosses to R26-TdTomato mice. Additional lineage tracing experiments were performed using Mist1-CreERT, Kitl-CreERT, Tff1-Cre, and Tff2-Cre mice crossed to reporter mice. Mice were given high-dose tamoxifen or DMP-777 or were infected with Helicobacter pylori to induce gastric metaplasia. We studied mice that expressed mutant forms of Ras in gastric cells, using TetO-KrasG12D, LSL-KrasG12D, and LSL-HrasG12V mice. We analyzed stomach tissues from GPR30-knockout mice. Mice were given dichloroacetate to inhibit pyruvate dehydrogenase kinase (PDK)-dependent cell competition.
RESULTS
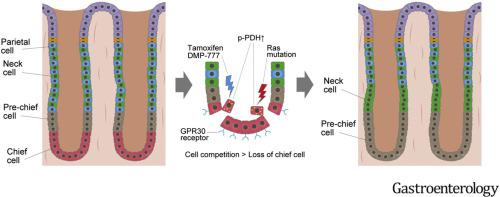
We identified GPR30, the G-protein-coupled form of the estrogen receptor, as a cell-specific marker of chief cells in gastric epithelium of mice. Gpr30-rtTA mice crossed to TetO-Cre;R26-TdTomato mice had specific expression of GPR30 in chief cells, with no expression noted in isthmus stem cells or lineage tracing of glands. Expression of mutant Kras in GPR30+ chief cells did not lead to the development of metaplasia or dysplasia but, instead, led to a reduction in labeled numbers of chief cells and a compensatory expansion of neck lineage, which was derived from upper Kitl+ clones. Administration of high-dose tamoxifen, DMP-777, or H pylori decreased the number of labeled chief cells. Chief cells were eliminated from epithelia via GPR30- and PDK-dependent cell competition after metaplastic stimuli, whereas loss of GRP30 or inhibition of PDK activity preserved chief cell numbers and attenuated neck lineage cell expansion.
CONCLUSIONS
In tracing studies of mice, we found that most chief cells are lost during metaplasia and therefore are unlikely to contribute to gastric carcinogenesis. Expansion of cells that coexpress neck and chief lineage markers, known as spasmolytic polypeptide-expressing metaplasia, does not occur via dedifferentiation from chief cells but, rather, through a compensatory response from neck progenitors to replace the eliminated chief cells.
中文翻译:

表达 GPR30 的胃主细胞不会去分化,而是在化生发展过程中通过 PDK 依赖性细胞竞争被消除。
背景与目的 胃主细胞是一种分泌消化酶的成熟细胞类型,已被认为是通过去分化或转分化发生化生和癌症的起源。然而,支持这一说法的研究存在技术局限性,包括主细胞标记物的特异性和所用药物的毒性等问题。因此,我们试图确定在主细胞中特异性表达的基因,并建立一个模型来追踪这些细胞。方法 我们对 Mist1-CreERT 追踪细胞进行了转录组分析,有或没有主细胞耗竭。生成 Gpr30-rtTA 小鼠并与 TetO-Cre 小鼠杂交,并在与 R26-TdTomato 小鼠杂交后进行谱系追踪。使用 Mist1-CreERT、Kitl-CreERT、Tff1-Cre、和 Tff2-Cre 小鼠与报告小鼠杂交。小鼠给予高剂量他莫昔芬或DMP-777或感染幽门螺杆菌诱导胃化生。我们使用 TetO-KrasG12D、LSL-KrasG12D 和 LSL-HrasG12V 小鼠研究了在胃细胞中表达 Ras 突变形式的小鼠。我们分析了 GPR30 敲除小鼠的胃组织。给予小鼠二氯乙酸以抑制丙酮酸脱氢酶激酶 (PDK) 依赖性细胞竞争。结果 我们将雌激素受体的 G 蛋白偶联形式 GPR30 鉴定为小鼠胃上皮细胞中主细胞的细胞特异性标记物。Gpr30-rtTA 小鼠与 TetO-Cre 杂交;R26-TdTomato 小鼠在主细胞中具有 GPR30 的特异性表达,在峡部干细胞或腺体谱系追踪中未发现表达。GPR30+ 主细胞中突变体 Kras 的表达不会导致化生或异型增生的发展,而是导致标记的主细胞数量减少和颈部谱系的代偿性扩张,这源自上层 Kitl+ 克隆。施用高剂量他莫昔芬、DMP-777 或幽门螺杆菌可减少标记主细胞的数量。在化生刺激后,主细胞通过 GPR30 和 PDK 依赖性细胞竞争从上皮细胞中消除,而 GRP30 的缺失或 PDK 活性的抑制保留了主细胞数量并减弱了颈部谱系细胞的扩张。结论 在对小鼠的追踪研究中,我们发现大多数主细胞在化生过程中丢失,因此不太可能导致胃癌发生。共表达颈部和主要谱系标记的细胞扩增,
更新日期:2020-02-04
中文翻译:

表达 GPR30 的胃主细胞不会去分化,而是在化生发展过程中通过 PDK 依赖性细胞竞争被消除。
背景与目的 胃主细胞是一种分泌消化酶的成熟细胞类型,已被认为是通过去分化或转分化发生化生和癌症的起源。然而,支持这一说法的研究存在技术局限性,包括主细胞标记物的特异性和所用药物的毒性等问题。因此,我们试图确定在主细胞中特异性表达的基因,并建立一个模型来追踪这些细胞。方法 我们对 Mist1-CreERT 追踪细胞进行了转录组分析,有或没有主细胞耗竭。生成 Gpr30-rtTA 小鼠并与 TetO-Cre 小鼠杂交,并在与 R26-TdTomato 小鼠杂交后进行谱系追踪。使用 Mist1-CreERT、Kitl-CreERT、Tff1-Cre、和 Tff2-Cre 小鼠与报告小鼠杂交。小鼠给予高剂量他莫昔芬或DMP-777或感染幽门螺杆菌诱导胃化生。我们使用 TetO-KrasG12D、LSL-KrasG12D 和 LSL-HrasG12V 小鼠研究了在胃细胞中表达 Ras 突变形式的小鼠。我们分析了 GPR30 敲除小鼠的胃组织。给予小鼠二氯乙酸以抑制丙酮酸脱氢酶激酶 (PDK) 依赖性细胞竞争。结果 我们将雌激素受体的 G 蛋白偶联形式 GPR30 鉴定为小鼠胃上皮细胞中主细胞的细胞特异性标记物。Gpr30-rtTA 小鼠与 TetO-Cre 杂交;R26-TdTomato 小鼠在主细胞中具有 GPR30 的特异性表达,在峡部干细胞或腺体谱系追踪中未发现表达。GPR30+ 主细胞中突变体 Kras 的表达不会导致化生或异型增生的发展,而是导致标记的主细胞数量减少和颈部谱系的代偿性扩张,这源自上层 Kitl+ 克隆。施用高剂量他莫昔芬、DMP-777 或幽门螺杆菌可减少标记主细胞的数量。在化生刺激后,主细胞通过 GPR30 和 PDK 依赖性细胞竞争从上皮细胞中消除,而 GRP30 的缺失或 PDK 活性的抑制保留了主细胞数量并减弱了颈部谱系细胞的扩张。结论 在对小鼠的追踪研究中,我们发现大多数主细胞在化生过程中丢失,因此不太可能导致胃癌发生。共表达颈部和主要谱系标记的细胞扩增,











































 京公网安备 11010802027423号
京公网安备 11010802027423号