Dyes and Pigments ( IF 4.1 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.dyepig.2020.108254 Dong-Jin Park , Puttavva Meti , Young-Dae Gong
|
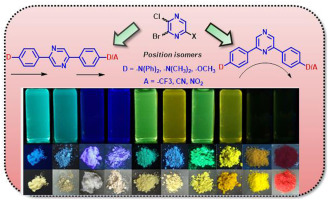
A versatile and expeditious synthetic route to pyrazine-based symmetric and asymmetric chromophores decorated with donor-acceptor (D-A) has been designed to study their structural effects on optical properties. Suzuki-Miyaura coupling of dihalopyrazine with various aryl boronic acids was synthesized under microwave condition. Pyrazine functionalized at C-2, C-5 and C-6 serve as acceptor to construct linear and angular push-pull chromophores. The photophysical, electrochemical and thermal properties of all the target chromophores were systematically investigated and the results were correlated theoretically by density functional theory computations. The emission wavelength was significantly red-shifted by introduction of strong electron withdrawing group (CN). The permutation of terminal donor acceptor units tunes the optoelectronic properties in a predictable way, aiding in the rational design of small molecule for luminescent materials. These chromophores displayed multicolour change in different solvents, exhibiting good solvatochromism with a large Stokes shift.
中文翻译:

基于二芳基吡嗪的位置异构体:光学性质和结构-性质关系的详细研究
设计了一种通用且快速的合成路线,以吡嗪为基的对称和不对称生色团装饰有供体-受体(DA),以研究其对光学性质的结构影响。在微波条件下合成了二卤代吡嗪与各种芳基硼酸的Suzuki-Miyaura偶联。在C-2,C-5和C-6上官能化的吡嗪用作受体,以构建线性和角推挽生色团。系统地研究了所有目标发色团的光物理,电化学和热性质,并通过密度泛函理论计算将结果与理论相关。通过引入强电子吸收基团(CN),发射波长显着红移。末端供体受体单元的排列以可预测的方式调节光电性能,有助于合理设计发光材料的小分子。这些生色团在不同的溶剂中表现出多色变化,表现出良好的溶剂变色性,斯托克斯位移较大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号