当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomically Dispersed Single Ni Site Catalysts for Nitrogen Reduction toward Electrochemical Ammonia Synthesis Using N2 and H2O
Small Methods ( IF 10.7 ) Pub Date : 2020-02-05 , DOI: 10.1002/smtd.201900821 Shreya Mukherjee 1 , Xiaoxuan Yang 1 , Weitao Shan 2 , Widitha Samarakoon 3 , Stavros Karakalos 4 , David A. Cullen 5 , Karren More 5 , Maoyu Wang 3 , Zhenxing Feng 3 , Guofeng Wang 2 , Gang Wu 1
Small Methods ( IF 10.7 ) Pub Date : 2020-02-05 , DOI: 10.1002/smtd.201900821 Shreya Mukherjee 1 , Xiaoxuan Yang 1 , Weitao Shan 2 , Widitha Samarakoon 3 , Stavros Karakalos 4 , David A. Cullen 5 , Karren More 5 , Maoyu Wang 3 , Zhenxing Feng 3 , Guofeng Wang 2 , Gang Wu 1
Affiliation
|
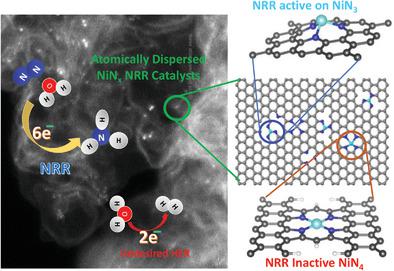
Ammonia (NH3) electrosynthesis gains significant attention as NH3 is essentially important for fertilizer production and fuel utilization. However, electrochemical nitrogen reduction reaction (NRR) remains a great challenge because of low activity and poor selectivity. Herein, a new class of atomically dispersed Ni site electrocatalyst is reported, which exhibits the optimal NH3 yield of 115 µg cm−2 h−1 at –0.8 V versus reversible hydrogen electrode (RHE) under neutral conditions. High faradic efficiency of 21 ± 1.9% is achieved at ‐0.2 V versus RHE under alkaline conditions, although the ammonia yield is lower. The Ni sites are stabilized with nitrogen, which is verified by advanced X‐ray absorption spectroscopy and electron microscopy. Density functional theory calculations provide insightful understanding on the possible structure of active sites, relevant reaction pathways, and confirm that the Ni‐N3 sites are responsible for the experimentally observed activity and selectivity. Extensive controls strongly suggest that the atomically dispersed NiN3 site‐rich catalyst provides more intrinsically active sites than those in N‐doped carbon, instead of possible environmental contamination. This work further indicates that single‐metal site catalysts with optimal nitrogen coordination is very promising for NRR and indeed improves the scaling relationship of transition metals.
中文翻译:

原子分散的单Ni中心催化剂,用于使用N2和H2O将氮还原为电化学合成氨
氨(NH 3)电合成受到了广泛关注,因为NH 3对于肥料生产和燃料利用至关重要。然而,由于活性低和选择性差,电化学氮还原反应(NRR)仍然是一个巨大的挑战。在此,报道了一种新型的原子分散的Ni中心电催化剂,其显示的最佳NH 3产量为115 µg cm -2 h -1与中性条件下的可逆氢电极(RHE)在–0.8 V下。相对于碱性条件下的RHE,在-0.2 V时,法拉第效率高达21±1.9%,尽管氨的产率较低。Ni的位点用氮稳定,这已通过先进的X射线吸收光谱法和电子显微镜证实。密度泛函理论计算可对活性位点的可能结构,相关的反应途径提供深刻的了解,并确认Ni-N 3位点是实验观察到的活性和选择性的原因。广泛的控制强烈表明原子分散的NiN 3富位催化剂比氮掺杂碳提供更多的固有活性位,而不是可能的环境污染。这项工作进一步表明,具有最佳氮配位的单金属中心催化剂对于NRR非常有前途,并且确实改善了过渡金属的结垢关系。
更新日期:2020-02-05
中文翻译:

原子分散的单Ni中心催化剂,用于使用N2和H2O将氮还原为电化学合成氨
氨(NH 3)电合成受到了广泛关注,因为NH 3对于肥料生产和燃料利用至关重要。然而,由于活性低和选择性差,电化学氮还原反应(NRR)仍然是一个巨大的挑战。在此,报道了一种新型的原子分散的Ni中心电催化剂,其显示的最佳NH 3产量为115 µg cm -2 h -1与中性条件下的可逆氢电极(RHE)在–0.8 V下。相对于碱性条件下的RHE,在-0.2 V时,法拉第效率高达21±1.9%,尽管氨的产率较低。Ni的位点用氮稳定,这已通过先进的X射线吸收光谱法和电子显微镜证实。密度泛函理论计算可对活性位点的可能结构,相关的反应途径提供深刻的了解,并确认Ni-N 3位点是实验观察到的活性和选择性的原因。广泛的控制强烈表明原子分散的NiN 3富位催化剂比氮掺杂碳提供更多的固有活性位,而不是可能的环境污染。这项工作进一步表明,具有最佳氮配位的单金属中心催化剂对于NRR非常有前途,并且确实改善了过渡金属的结垢关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号