当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C-N Coupling of DNA-Conjugated (Hetero)aryl Bromides and Chlorides for DNA-Encoded Chemical Library Synthesis.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.bioconjchem.9b00863 Ying-Chu Chen 1 , John C Faver 1 , Angela F Ku 1 , Gabriella Miklossy 1 , Kevin Riehle 1 , Kurt M Bohren 1 , Melek N Ucisik 1 , Martin M Matzuk 1 , Zhifeng Yu 1 , Nicholas Simmons 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.bioconjchem.9b00863 Ying-Chu Chen 1 , John C Faver 1 , Angela F Ku 1 , Gabriella Miklossy 1 , Kevin Riehle 1 , Kurt M Bohren 1 , Melek N Ucisik 1 , Martin M Matzuk 1 , Zhifeng Yu 1 , Nicholas Simmons 1
Affiliation
|
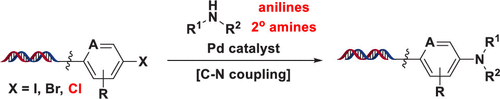
DNA-encoded chemical library (DECL) screens are a rapid and economical tool to identify chemical starting points for drug discovery. As a robust transformation for drug discovery, palladium-catalyzed C-N coupling is a valuable synthetic method for the construction of DECL chemical matter; however, currently disclosed methods have only been demonstrated on DNA-attached (hetero)aromatic iodide and bromide electrophiles. We developed conditions utilizing an N-heterocyclic carbene-palladium catalyst that extends this reaction to the coupling of DNA-conjugated (hetero)aromatic chlorides with (hetero)aromatic and select aliphatic amine nucleophiles. In addition, we evaluated steric and electronic effects within this catalyst series, carried out a large substrate scope study on two representative (hetero)aryl bromides, and applied this newly developed method within the construction of a 63 million-membered DECL.
中文翻译:

DNA缀合的(杂)芳基溴化物和氯化物的CN偶联,用于DNA编码的化学文库合成。
DNA编码的化学文库(DECL)筛选是一种快速经济的工具,可识别用于药物发现的化学起点。钯催化的CN偶联作为一种强大的药物发现转化方法,是构建DECL化学物质的有价值的合成方法。然而,目前公开的方法仅在附有DNA的(杂)芳族碘化物和溴化物亲电试剂上得到证实。我们开发了利用N-杂环卡宾-钯催化剂的条件,该催化剂将这一反应扩展到DNA共轭(杂)芳族氯化物与(杂)芳族和精选脂肪族胺亲核试剂的偶联。此外,我们评估了该催化剂系列中的空间和电子效应,对两种代表性的(杂)芳基溴化物进行了大范围的底物研究,
更新日期:2020-02-23
中文翻译:

DNA缀合的(杂)芳基溴化物和氯化物的CN偶联,用于DNA编码的化学文库合成。
DNA编码的化学文库(DECL)筛选是一种快速经济的工具,可识别用于药物发现的化学起点。钯催化的CN偶联作为一种强大的药物发现转化方法,是构建DECL化学物质的有价值的合成方法。然而,目前公开的方法仅在附有DNA的(杂)芳族碘化物和溴化物亲电试剂上得到证实。我们开发了利用N-杂环卡宾-钯催化剂的条件,该催化剂将这一反应扩展到DNA共轭(杂)芳族氯化物与(杂)芳族和精选脂肪族胺亲核试剂的偶联。此外,我们评估了该催化剂系列中的空间和电子效应,对两种代表性的(杂)芳基溴化物进行了大范围的底物研究,











































 京公网安备 11010802027423号
京公网安备 11010802027423号