当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Soybean Lipoxygenase-Substrate Complex: Correlation between the Properties of Tunneling-Ready States and ENDOR-Detected Structures of Ground States.
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-05 , DOI: 10.1021/acs.biochem.9b00861 Adam R Offenbacher 1, 2 , Ajay Sharma 3 , Peter E Doan 3 , Judith P Klinman 2, 4 , Brian M Hoffman 3, 5
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-05 , DOI: 10.1021/acs.biochem.9b00861 Adam R Offenbacher 1, 2 , Ajay Sharma 3 , Peter E Doan 3 , Judith P Klinman 2, 4 , Brian M Hoffman 3, 5
Affiliation
|
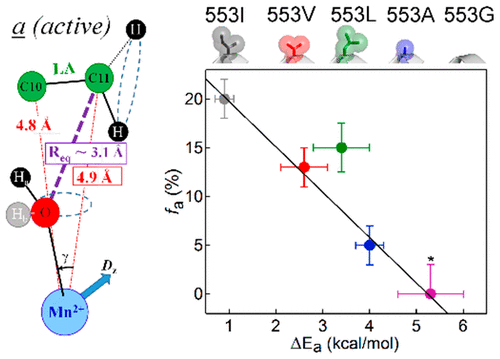
Hydrogen tunneling in enzymatic C-H activation requires a dynamical sampling among ground-state enzyme-substrate (E-S) conformations, which transiently generates a tunneling-ready state (TRS). The TRS is characterized by a hydrogen donor-acceptor distance (DAD) of 2.7 Å, ∼0.5 Å shorter than the dominant DAD of optimized ground states. Recently, a high-resolution, 13C electron-nuclear double-resonance (ENDOR) approach was developed to characterize the ground-state structure of the complex of the linoleic acid (LA) substrate with soybean lipoxygenase (SLO). The resulting enzyme-substrate model revealed two ground-state conformers with different distances between the target C11 of LA and the catalytically active cofactor [Fe(III)-OH]: the active conformer "a", with a van der Waals DAD of 3.1 Å between C11 and metal-bound hydroxide, and an inactive conformer "b", with a distance that is almost 1 Å longer. Herein, the structure of the E-S complex is examined for a series of six variants in which subtle structural modifications of SLO have been introduced either at a hydrophobic side chain near the bound substrate or at a remote residue within a protein network whose flexibility influences hydrogen transfer. A remarkable correlation is found between the ENDOR-derived population of the active ground-state conformer a and the kinetically derived differential enthalpic barrier for D versus H transfer, ΔEa, with the latter increasing as the fraction of conformer a decreases. As proposed, ΔEa provides a "ruler" for the DAD within the TRS. ENDOR measurements further corroborate the previous identification of a dynamical network coupling the buried active site of SLO to the surface. This study shows that subtle imperfections within the initial ground-state structures of E-S complexes are accompanied by compromised geometries at the TRS.
中文翻译:

大豆脂氧合酶-底物复合物:隧道就绪态的特性与 ENDOR 检测到的基态结构之间的相关性。
酶促 CH 激活中的氢隧道效应需要在基态酶-底物 (ES) 构象中进行动态采样,从而瞬时产生隧道效应就绪状态 (TRS)。 TRS 的特点是氢供体-受体距离 (DAD) 为 2.7 Å,比优化基态的主要 DAD 短约 0.5 Å。最近,开发了一种高分辨率 13C 电子核双共振 (ENDOR) 方法来表征亚油酸 (LA) 底物与大豆脂氧合酶 (SLO) 复合物的基态结构。由此产生的酶-底物模型揭示了两种基态构象异构体,它们在 LA 的目标 C11 和催化活性辅因子 [Fe(III)-OH] 之间具有不同的距离:活性构象异构体“a”,范德华 DAD 为 3.1 C11 和金属结合氢氧化物之间的 Å,以及非活性构象异构体“b”,距离几乎长了 1 Å。在此,检查了 ES 复合物的结构是否有一系列六种变体,其中在靠近结合底物的疏水侧链或在蛋白质网络内的远程残基处引入了 SLO 的微妙结构修饰,其灵活性影响氢转移。在活性基态构象异构体 a 的 ENDOR 衍生群体与动力学衍生的 D 与 H 转移的差动焓垒 ΔEa 之间发现了显着的相关性,其中后者随着构象异构体 a 分数的减少而增加。正如所提出的,ΔEa 为 TRS 内的 DAD 提供了一个“标尺”。 ENDOR 测量进一步证实了先前对 SLO 埋藏活性位点与地表耦合动力网络的识别。 这项研究表明,ES 复合物初始基态结构中的细微缺陷伴随着 TRS 几何形状的受损。
更新日期:2020-02-06
中文翻译:

大豆脂氧合酶-底物复合物:隧道就绪态的特性与 ENDOR 检测到的基态结构之间的相关性。
酶促 CH 激活中的氢隧道效应需要在基态酶-底物 (ES) 构象中进行动态采样,从而瞬时产生隧道效应就绪状态 (TRS)。 TRS 的特点是氢供体-受体距离 (DAD) 为 2.7 Å,比优化基态的主要 DAD 短约 0.5 Å。最近,开发了一种高分辨率 13C 电子核双共振 (ENDOR) 方法来表征亚油酸 (LA) 底物与大豆脂氧合酶 (SLO) 复合物的基态结构。由此产生的酶-底物模型揭示了两种基态构象异构体,它们在 LA 的目标 C11 和催化活性辅因子 [Fe(III)-OH] 之间具有不同的距离:活性构象异构体“a”,范德华 DAD 为 3.1 C11 和金属结合氢氧化物之间的 Å,以及非活性构象异构体“b”,距离几乎长了 1 Å。在此,检查了 ES 复合物的结构是否有一系列六种变体,其中在靠近结合底物的疏水侧链或在蛋白质网络内的远程残基处引入了 SLO 的微妙结构修饰,其灵活性影响氢转移。在活性基态构象异构体 a 的 ENDOR 衍生群体与动力学衍生的 D 与 H 转移的差动焓垒 ΔEa 之间发现了显着的相关性,其中后者随着构象异构体 a 分数的减少而增加。正如所提出的,ΔEa 为 TRS 内的 DAD 提供了一个“标尺”。 ENDOR 测量进一步证实了先前对 SLO 埋藏活性位点与地表耦合动力网络的识别。 这项研究表明,ES 复合物初始基态结构中的细微缺陷伴随着 TRS 几何形状的受损。











































 京公网安备 11010802027423号
京公网安备 11010802027423号