当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gestational diabetes impacts fetal precursor cell responses with potential consequences for offspring.
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2019-12-27 , DOI: 10.1002/sctm.19-0242 Francisco Algaba-Chueca 1, 2, 3 , Elsa Maymó-Masip 1, 2, 3 , Miriam Ejarque 1, 2, 3 , Mónica Ballesteros 2, 4 , Gemma Llauradó 3, 5 , Carlos López 2, 6 , Albert Guarque 2, 4 , Carolina Serena 1, 2, 3 , Laia Martínez-Guasch 1, 2, 3 , Cristina Gutiérrez 1, 2 , Ramón Bosch 2, 6 , Joan Vendrell 1, 2, 3, 7 , Ana Megía 1, 2, 3, 7 , Sonia Fernández-Veledo 1, 2, 3
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2019-12-27 , DOI: 10.1002/sctm.19-0242 Francisco Algaba-Chueca 1, 2, 3 , Elsa Maymó-Masip 1, 2, 3 , Miriam Ejarque 1, 2, 3 , Mónica Ballesteros 2, 4 , Gemma Llauradó 3, 5 , Carlos López 2, 6 , Albert Guarque 2, 4 , Carolina Serena 1, 2, 3 , Laia Martínez-Guasch 1, 2, 3 , Cristina Gutiérrez 1, 2 , Ramón Bosch 2, 6 , Joan Vendrell 1, 2, 3, 7 , Ana Megía 1, 2, 3, 7 , Sonia Fernández-Veledo 1, 2, 3
Affiliation
|
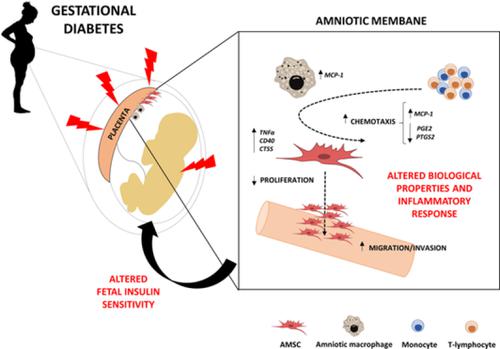
Fetal programming has been proposed as a key mechanism underlying the association between intrauterine exposure to maternal diabetes and negative health outcomes in offspring. To determine whether gestational diabetes mellitus (GDM) might leave an imprint in fetal precursors of the amniotic membrane and whether it might be related to adverse outcomes in offspring, a prospective case‐control study was conducted, in which amniotic mesenchymal stem cells (AMSCs) and resident macrophages were isolated from pregnant patients, with either GDM or normal glucose tolerance, scheduled for cesarean section. After characterization, functional characteristics of AMSCs were analyzed and correlated with anthropometrical and clinical variables from both mother and offspring. GDM‐derived AMSCs displayed an impaired proliferation and osteogenic potential when compared with control cells, accompanied by superior invasive and chemotactic capacity. The expression of genes involved in the inflammatory response (TNFα, MCP‐1, CD40, and CTSS) was upregulated in GDM‐derived AMSCs, whereas anti‐inflammatory IL‐33 was downregulated. Macrophages isolated from the amniotic membrane of GDM mothers consistently showed higher expression of MCP‐1 as well. In vitro studies in which AMSCs from healthy control women were exposed to hyperglycemia, hyperinsulinemia, and palmitic acid confirmed these results. Finally, genes involved in the inflammatory response were associated with maternal insulin sensitivity and prepregnancy body mass index, as well as with fetal metabolic parameters. These results suggest that the GDM environment could program stem cells and subsequently favor metabolic dysfunction later in life. Fetal adaptive programming in the setting of GDM might have a direct negative impact on insulin resistance of offspring.
中文翻译:

妊娠糖尿病会影响胎儿前体细胞反应,并对后代产生潜在影响。
胎儿编程已被提出为子宫内暴露于母体糖尿病与后代健康状况不良之间关联的关键机制。为了确定妊娠糖尿病(GDM)是否可能在羊膜的胎儿前体上留下印记,以及它是否可能与后代的不良结局有关,进行了一项前瞻性病例对照研究,其中羊膜间充质干细胞(AMSC)并从妊娠患者中分离出具有GDM或葡萄糖耐量正常的巨噬细胞,并计划行剖宫产。表征后,分析了AMSC的功能特性,并将其与来自母亲和后代的人体测量学和临床变量相关联。与对照细胞相比,源自GDM的AMSC表现出增殖能力和成骨能力受损,并具有出色的侵袭和趋化能力。参与炎症反应的基因的表达(在GDM衍生的AMSC中,TNFα,MCP-1,CD40和CTSS)被上调,而抗炎性IL-33被下调。从GDM母亲的羊膜分离出的巨噬细胞始终显示出MCP-1的较高表达也一样 来自健康对照妇女的AMSC暴露于高血糖,高胰岛素血症和棕榈酸的体外研究证实了这些结果。最后,与炎症反应有关的基因与母亲的胰岛素敏感性和孕期体重指数以及胎儿的代谢参数有关。这些结果表明,GDM环境可以对干细胞进行编程,从而在以后的生活中有利于代谢功能障碍。GDM情况下的胎儿适应性编程可能会对后代的胰岛素抵抗产生直接的负面影响。
更新日期:2019-12-27
中文翻译:

妊娠糖尿病会影响胎儿前体细胞反应,并对后代产生潜在影响。
胎儿编程已被提出为子宫内暴露于母体糖尿病与后代健康状况不良之间关联的关键机制。为了确定妊娠糖尿病(GDM)是否可能在羊膜的胎儿前体上留下印记,以及它是否可能与后代的不良结局有关,进行了一项前瞻性病例对照研究,其中羊膜间充质干细胞(AMSC)并从妊娠患者中分离出具有GDM或葡萄糖耐量正常的巨噬细胞,并计划行剖宫产。表征后,分析了AMSC的功能特性,并将其与来自母亲和后代的人体测量学和临床变量相关联。与对照细胞相比,源自GDM的AMSC表现出增殖能力和成骨能力受损,并具有出色的侵袭和趋化能力。参与炎症反应的基因的表达(在GDM衍生的AMSC中,TNFα,MCP-1,CD40和CTSS)被上调,而抗炎性IL-33被下调。从GDM母亲的羊膜分离出的巨噬细胞始终显示出MCP-1的较高表达也一样 来自健康对照妇女的AMSC暴露于高血糖,高胰岛素血症和棕榈酸的体外研究证实了这些结果。最后,与炎症反应有关的基因与母亲的胰岛素敏感性和孕期体重指数以及胎儿的代谢参数有关。这些结果表明,GDM环境可以对干细胞进行编程,从而在以后的生活中有利于代谢功能障碍。GDM情况下的胎儿适应性编程可能会对后代的胰岛素抵抗产生直接的负面影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号