Protein & Peptide Letters ( IF 1.6 ) Pub Date : 2020-05-31 , DOI: 10.2174/0929866527666191230103739 Victor Teatini Ribeiro 1 , Leonardo Cruz de Souza 1, 2 , Ana Cristina Simões E Silva 1
|
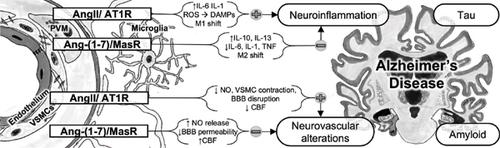
New roles of the Renin-Angiotensin System (RAS), apart from fluid homeostasis and Blood Pressure (BP) regulation, are being progressively unveiled, since the discoveries of RAS alternative axes and local RAS in different tissues, including the brain. Brain RAS is reported to interact with pathophysiological mechanisms of many neurological and psychiatric diseases, including Alzheimer’s Disease (AD). Even though AD is the most common cause of dementia worldwide, its pathophysiology is far from elucidated. Currently, no treatment can halt the disease course. Successive failures of amyloid-targeting drugs have challenged the amyloid hypothesis and increased the interest in the inflammatory and vascular aspects of AD. RAS compounds, both centrally and peripherally, potentially interact with neuroinflammation and cerebrovascular regulation. This narrative review discusses the AD pathophysiology and its possible interaction with RAS, looking forward to potential therapeutic approaches. RAS molecules affect BP, cerebral blood flow, neuroinflammation, and oxidative stress. Angiotensin (Ang) II, via angiotensin type 1 receptors may promote brain tissue damage, while Ang-(1-7) seems to elicit neuroprotection. Several studies dosed RAS molecules in AD patients' biological material, with heterogeneous results. The link between AD and clinical conditions related to classical RAS axis overactivation (hypertension, heart failure, and chronic kidney disease) supports the hypothesized role of this system in AD. Additionally, RAStargeting drugs as Angiotensin Converting Enzyme inhibitors (ACEis) and Angiotensin Receptor Blockers (ARBs) seem to exert beneficial effects on AD. Results of randomized controlled trials testing ACEi or ARBs in AD are awaited to elucidate whether AD-RAS interaction has implications on AD therapeutics.
中文翻译:

肾素-血管紧张素系统和阿尔茨海默氏病的病理生理学:从潜在的相互作用到治疗的观点。
肾素-血管紧张素系统(RAS)的新作用,除了流体稳态和血压(BP)调节外,还逐渐被揭示,因为在包括大脑在内的不同组织中发现了RAS替代轴和局部RAS。据报道,脑RAS与许多神经和精神疾病(包括阿尔茨海默氏病(AD))的病理生理机制相互作用。尽管AD是全世界痴呆症最常见的病因,但其病理生理学还远未阐明。目前,没有任何治疗方法可以阻止疾病进程。靶向淀粉样蛋白的药物的连续失败挑战了淀粉样蛋白的假设,并增加了对AD炎症和血管方面的兴趣。RAS化合物在中心和外围均可能与神经炎症和脑血管调节相互作用。这篇叙述性评论讨论了AD病理生理学及其与RAS的可能相互作用,并期待潜在的治疗方法。RAS分子会影响血压,脑血流量,神经炎症和氧化应激。血管紧张素(Ang)II,通过1型血管紧张素受体可能促进脑组织损伤,而Ang-(1-7)似乎引起神经保护作用。几项研究在AD患者的生物材料中使用了RAS分子,结果却不一致。AD与与经典RAS轴过度激活有关的临床状况(高血压,心力衰竭和慢性肾脏疾病)之间的联系支持了该系统在AD中的假设作用。此外,RAS靶向药物,如血管紧张素转换酶抑制剂(ACEis)和血管紧张素受体阻滞剂(ARB)似乎对AD发挥有益作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号