当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbamylation reduces the capacity of IgG for hexamerization and complement activation.
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-01-05 , DOI: 10.1111/cei.13411 R Lubbers 1 , S C Oostindie 2, 3 , D J Dijkstra 3 , P W H I Parren 3, 4 , M K Verheul 1 , L Abendstein 5 , T H Sharp 5 , A de Ru 6 , G M C Janssen 6 , P A van Veelen 6 , E T J van den Bremer 2 , B Bleijlevens 2 , B-J de Kreuk 2 , F J Beurskens 2 , L A Trouw 1, 3
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-01-05 , DOI: 10.1111/cei.13411 R Lubbers 1 , S C Oostindie 2, 3 , D J Dijkstra 3 , P W H I Parren 3, 4 , M K Verheul 1 , L Abendstein 5 , T H Sharp 5 , A de Ru 6 , G M C Janssen 6 , P A van Veelen 6 , E T J van den Bremer 2 , B Bleijlevens 2 , B-J de Kreuk 2 , F J Beurskens 2 , L A Trouw 1, 3
Affiliation
|
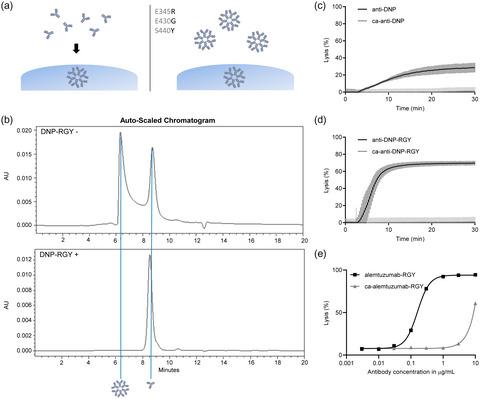
Carbamylation is a post‐translational modification that can be detected on a range of proteins, including immunoglobulin (Ig)G, in several clinical conditions. Carbamylated IgG (ca‐IgG) was reported to lose its capacity to trigger complement activation, but the mechanism remains unclear. Because C1q binds with high affinity to hexameric IgG, we analyzed whether carbamylation of IgG affects binding of C1q, hexamerization and complement‐dependent cytotoxicity (CDC). Synovial tissues of rheumatoid arthritis (RA) patients were analyzed for the presence of ca‐IgG in vivo. Synovial tissues from RA patients were analyzed for the presence of ca‐IgG using mass spectrometry (MS). Monomeric or hexameric antibodies were carbamylated in vitro and quality in solution was controlled. The capacity of ca‐IgG to activate complement was analyzed in enzyme‐linked immunosorbent (ELISAs) and cellular CDC assays. Using MS, we identified ca‐IgG to be present in the joints of RA patients. Using in vitro carbamylated antibodies, we observed that ca‐IgG lost its capacity to activate complement in both solid‐phase and CDC assays. Mixing ca‐IgG with non‐modified IgG did not result in effective inhibition of complement activation by ca‐IgG. Carbamylation of both monomeric IgG and preformed hexameric IgG greatly impaired the capacity to trigger complement activation. Furthermore, upon carbamylation, the preformed hexameric IgG dissociated into monomeric IgG in solution, indicating that carbamylation influences both hexamerization and C1q binding. In conclusion, ca‐IgG can be detected in vivo and has a strongly reduced capacity to activate complement which is, in part, mediated through a reduced ability to form hexamers.
中文翻译:

氨基甲酸酯化降低了IgG六聚化和补体激活的能力。
氨基甲酸酯化是一种翻译后修饰,可以在多种临床情况下在多种蛋白质上检测到,包括免疫球蛋白(Ig)G。据报道,氨基甲酸酯化的IgG(ca-IgG)失去了触发补体激活的能力,但机理尚不清楚。由于C1q与六聚体IgG具有高亲和力结合,因此我们分析了IgG的氨甲酰化是否会影响C1q的结合,六聚化和补体依赖性细胞毒性(CDC)。对类风湿关节炎(RA)患者的滑膜组织进行体内ca-IgG分析。使用质谱(MS)分析RA患者的滑膜组织中是否存在ca‐IgG。单体或六聚体抗体在体外被氨甲酰化并控制溶液质量。通过酶联免疫吸附(ELISA)和细胞CDC测定法分析了ca-IgG激活补体的能力。使用MS,我们确定了RA患者关节中存在ca-IgG。体外使用氨甲酰化抗体,我们观察到ca-IgG在固相和CDC分析中均丧失了激活补体的能力。将ca-IgG与未修饰的IgG混合不会导致ca-IgG有效抑制补体激活。单体IgG和预先形成的六聚体IgG的氨基甲酸酯化大大削弱了触发补体激活的能力。此外,在氨基甲酸酯化时,预先形成的六聚体IgG在溶液中解离成单体IgG,表明氨基甲酸酯化影响六聚化和C1q结合。总之,可以在体内检测到ca-IgG ,并且其激活补体的能力大大降低,这部分是由形成六聚体的能力降低所介导的。
更新日期:2020-03-26
中文翻译:

氨基甲酸酯化降低了IgG六聚化和补体激活的能力。
氨基甲酸酯化是一种翻译后修饰,可以在多种临床情况下在多种蛋白质上检测到,包括免疫球蛋白(Ig)G。据报道,氨基甲酸酯化的IgG(ca-IgG)失去了触发补体激活的能力,但机理尚不清楚。由于C1q与六聚体IgG具有高亲和力结合,因此我们分析了IgG的氨甲酰化是否会影响C1q的结合,六聚化和补体依赖性细胞毒性(CDC)。对类风湿关节炎(RA)患者的滑膜组织进行体内ca-IgG分析。使用质谱(MS)分析RA患者的滑膜组织中是否存在ca‐IgG。单体或六聚体抗体在体外被氨甲酰化并控制溶液质量。通过酶联免疫吸附(ELISA)和细胞CDC测定法分析了ca-IgG激活补体的能力。使用MS,我们确定了RA患者关节中存在ca-IgG。体外使用氨甲酰化抗体,我们观察到ca-IgG在固相和CDC分析中均丧失了激活补体的能力。将ca-IgG与未修饰的IgG混合不会导致ca-IgG有效抑制补体激活。单体IgG和预先形成的六聚体IgG的氨基甲酸酯化大大削弱了触发补体激活的能力。此外,在氨基甲酸酯化时,预先形成的六聚体IgG在溶液中解离成单体IgG,表明氨基甲酸酯化影响六聚化和C1q结合。总之,可以在体内检测到ca-IgG ,并且其激活补体的能力大大降低,这部分是由形成六聚体的能力降低所介导的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号