当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A pump-free tricellular blood-brain barrier on-a-chip model to understand barrier property and evaluate drug response.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-01-18 , DOI: 10.1002/bit.27260 Fang Yu 1 , Nivasini D/O Selva Kumar 2 , Lynette C Foo 2 , Sum Huan Ng 1 , Walter Hunziker 2, 3 , Deepak Choudhury 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-01-18 , DOI: 10.1002/bit.27260 Fang Yu 1 , Nivasini D/O Selva Kumar 2 , Lynette C Foo 2 , Sum Huan Ng 1 , Walter Hunziker 2, 3 , Deepak Choudhury 1
Affiliation
|
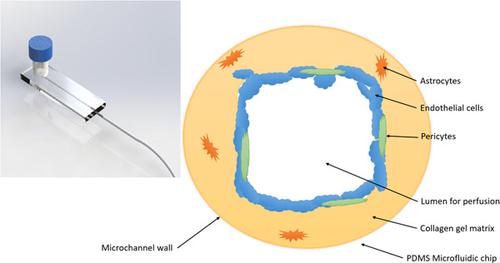
Disruption of the blood–brain barrier (BBB) leads to various neurovascular diseases. Development of therapeutics required to cross the BBB is difficult due to a lack of relevant in vitro models. We have developed a three‐dimensional (3D) microfluidic BBB chip (BBBC) to study cell interactions in the brain microvasculature and to test drug candidates of neurovascular diseases. We isolated primary brain microvascular endothelial cells (ECs), pericytes, and astrocytes from neonatal rats and cocultured them in the BBBC. To mimic the 3D in vivo BBB structure, we used type I collagen hydrogel to pattern the microchannel via viscous finger patterning technique to create a matrix. ECs, astrocytes, and pericytes were cocultured in the collagen matrix. The fluid flow in the BBBC was controlled by a pump‐free strategy utilizing gravity as driving force and resistance in a paper‐based flow resistor. The primary cells cultured in the BBBC expressed high levels of junction proteins and formed a tight endothelial barrier layer. Addition of tumor necrosis factor alpha to recapitulate neuroinflammatory conditions compromised the BBB functionality. To mitigate the neuroinflammatory stimulus, we treated the BBB model with the glucocorticoid drug dexamethasone, and observed protection of the BBB. This BBBC represents a new simple, cost‐effective, and scalable in vitro platform for validating therapeutic drugs targeting neuroinflammatory conditions.
中文翻译:

无泵三细胞血脑屏障芯片模型可了解屏障特性并评估药物反应。
血脑屏障(BBB)的破坏导致各种神经血管疾病。由于缺乏相关的体外模型,很难开发出穿越BBB所需的疗法。我们已经开发了三维(3D)微流控BBB芯片(BBBC),以研究大脑微脉管系统中的细胞相互作用并测试神经血管疾病的候选药物。我们从新生大鼠中分离了原代的大脑微血管内皮细胞(EC),周细胞和星形胶质细胞,并将它们在BBBC中共培养。为了模拟3D体内BBB结构,我们使用I型胶原蛋白水凝胶通过粘性手指构图技术构图微通道,以创建基质。EC,星形胶质细胞和周细胞在胶原蛋白基质中共培养。BBBC中的流体流量是通过无泵策略控制的,该策略利用重力作为驱动力和纸基流阻中的阻力。在BBBC中培养的原代细胞表达高水平的连接蛋白,并形成紧密的内皮屏障层。增加肿瘤坏死因子α来概括神经炎性疾病损害了血脑屏障功能。为了减轻神经炎症刺激,我们用糖皮质激素药物地塞米松治疗了BBB模型,并观察到对BBB的保护。该BBBC代表了一种新的简单,经济高效且可扩展的体外平台,用于验证针对神经炎性疾病的治疗药物。在BBBC中培养的原代细胞表达高水平的连接蛋白,并形成紧密的内皮屏障层。增加肿瘤坏死因子α来概括神经炎性疾病损害了血脑屏障功能。为了减轻神经炎症刺激,我们用糖皮质激素类药物地塞米松治疗了BBB模型,并观察到对BBB的保护。该BBBC代表了一种新的简单,经济高效且可扩展的体外平台,用于验证针对神经炎性疾病的治疗药物。在BBBC中培养的原代细胞表达高水平的连接蛋白,并形成紧密的内皮屏障层。增加肿瘤坏死因子α来概括神经炎性疾病损害了血脑屏障功能。为了减轻神经炎症刺激,我们用糖皮质激素药物地塞米松治疗了BBB模型,并观察到对BBB的保护。该BBBC代表了一种新的简单,经济高效且可扩展的体外平台,用于验证针对神经炎性疾病的治疗药物。
更新日期:2020-03-09
中文翻译:

无泵三细胞血脑屏障芯片模型可了解屏障特性并评估药物反应。
血脑屏障(BBB)的破坏导致各种神经血管疾病。由于缺乏相关的体外模型,很难开发出穿越BBB所需的疗法。我们已经开发了三维(3D)微流控BBB芯片(BBBC),以研究大脑微脉管系统中的细胞相互作用并测试神经血管疾病的候选药物。我们从新生大鼠中分离了原代的大脑微血管内皮细胞(EC),周细胞和星形胶质细胞,并将它们在BBBC中共培养。为了模拟3D体内BBB结构,我们使用I型胶原蛋白水凝胶通过粘性手指构图技术构图微通道,以创建基质。EC,星形胶质细胞和周细胞在胶原蛋白基质中共培养。BBBC中的流体流量是通过无泵策略控制的,该策略利用重力作为驱动力和纸基流阻中的阻力。在BBBC中培养的原代细胞表达高水平的连接蛋白,并形成紧密的内皮屏障层。增加肿瘤坏死因子α来概括神经炎性疾病损害了血脑屏障功能。为了减轻神经炎症刺激,我们用糖皮质激素药物地塞米松治疗了BBB模型,并观察到对BBB的保护。该BBBC代表了一种新的简单,经济高效且可扩展的体外平台,用于验证针对神经炎性疾病的治疗药物。在BBBC中培养的原代细胞表达高水平的连接蛋白,并形成紧密的内皮屏障层。增加肿瘤坏死因子α来概括神经炎性疾病损害了血脑屏障功能。为了减轻神经炎症刺激,我们用糖皮质激素类药物地塞米松治疗了BBB模型,并观察到对BBB的保护。该BBBC代表了一种新的简单,经济高效且可扩展的体外平台,用于验证针对神经炎性疾病的治疗药物。在BBBC中培养的原代细胞表达高水平的连接蛋白,并形成紧密的内皮屏障层。增加肿瘤坏死因子α来概括神经炎性疾病损害了血脑屏障功能。为了减轻神经炎症刺激,我们用糖皮质激素药物地塞米松治疗了BBB模型,并观察到对BBB的保护。该BBBC代表了一种新的简单,经济高效且可扩展的体外平台,用于验证针对神经炎性疾病的治疗药物。










































 京公网安备 11010802027423号
京公网安备 11010802027423号