当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phenotypic shift of small intestinal intra-epithelial type 1 innate lymphoid cells in celiac disease is associated with enhanced cytotoxic potential.
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-01-27 , DOI: 10.1111/cei.13414 M Uhde 1 , X Yu 1 , A Bunin 1 , C Brauner 1 , S K Lewis 1 , B Lebwohl 1 , S Krishnareddy 1 , A Alaedini 1, 2 , B Reizis 3 , S Ghosh 4 , P H Green 1 , G Bhagat 1, 5
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-01-27 , DOI: 10.1111/cei.13414 M Uhde 1 , X Yu 1 , A Bunin 1 , C Brauner 1 , S K Lewis 1 , B Lebwohl 1 , S Krishnareddy 1 , A Alaedini 1, 2 , B Reizis 3 , S Ghosh 4 , P H Green 1 , G Bhagat 1, 5
Affiliation
|
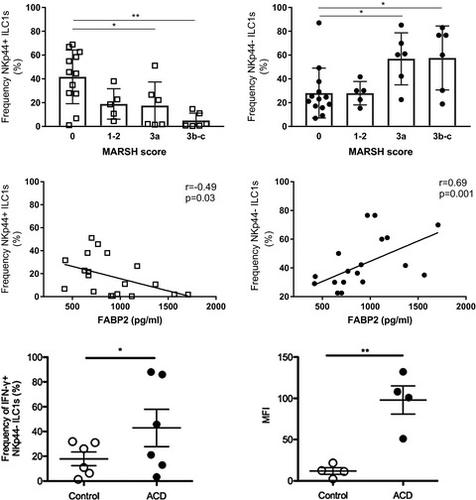
The small intestinal (SI) epithelium harbors a heterogeneous population of lymphocytes that mediate mucosal damage and repair in celiac disease (CD). The composition and roles of human proximal SI intra-epithelial innate lymphoid cells (ILCs), and their alterations in CD, are not well understood. We report that duodenal intra-epithelial ILCs predominantly consist of natural killer (NK)p44+ CD127- cytotoxic ILC1s and NKp44- CD127+ helper ILC1s, while ILC3s only represent a minor population. In patients with newly diagnosed or active CD (ACD) and refractory CD type 1 (RCD I), the frequency of SI NKp44+ ILCs is decreased, with restoration of NKp44+ ILC frequency observed in patients adhering to a gluten-free diet who show evidence of mucosal healing. Moreover, the frequency of SI NKp44- ILCs is increased in ACD and RCD I patients and correlates with the severity of villous atrophy and epithelial damage, as assessed by serum levels of fatty acid binding protein 2 (FABP2). We show that the ILC alterations in CD represent a phenotypic shift of cytotoxic ILC1s rather than an increase in helper ILC1s or transdifferentiation of ILC1s to ILC3s, and activation-induced loss of NKp44 by cytotoxic ILC1s is associated with increased interferon (IFN)-γ expression and release of lytic granules. These findings suggest that intra-epithelial NKp44- CD127- cytotoxic ILC1s may contribute to mucosal damage in CD.
中文翻译:

乳糜泻中小肠上皮内1型先天淋巴样细胞的表型转移与增强的细胞毒性潜力有关。
小肠(SI)上皮具有异质性淋巴细胞群,可在乳糜泻(CD)中介导粘膜损伤和修复。人类近端SI上皮内固有淋巴样细胞(ILC)的组成和作用,以及它们在CD中的变化,尚不十分清楚。我们报告十二指肠上皮内ILC主要由自然杀手(NK)p44 + CD127细胞毒性ILC1s和NKp44 CD127 +辅助ILC1s组成,而ILC3仅代表少数人群。在患有新诊断或活动性CD(ACD)和1型难治性CD(RCD I)的患者中,SI NKp44 + ILC的频率降低,坚持无麸质饮食并显示证据的NKp44 + ILC频率得以恢复。黏膜愈合。此外,SI NKp44-ILCs的频率在ACD和RCD I患者中增加,并与绒毛萎缩和上皮损害的严重程度相关,如通过血清脂肪酸结合蛋白2(FABP2)的水平评估。我们显示CD中的ILC改变代表细胞毒性ILC1的表型转变,而不是辅助ILC1s的增加或ILC1s向ILC3s的转分化,并且由细胞毒性ILC1s引起的NKp44激活诱导的丢失与干扰素(IFN)-γ表达增加相关释放溶菌颗粒。这些发现表明,上皮内NKp44-CD127-细胞毒性ILC1s可能导致CD的粘膜损伤。我们显示CD中的ILC改变代表细胞毒性ILC1的表型转变,而不是辅助ILC1s的增加或ILC1s向ILC3s的转分化,并且由细胞毒性ILC1s引起的NKp44激活诱导的丢失与干扰素(IFN)-γ表达增加相关释放溶菌颗粒。这些发现表明,上皮内NKp44-CD127-细胞毒性ILC1s可能导致CD的粘膜损伤。我们显示CD中的ILC改变代表细胞毒性ILC1的表型转变,而不是辅助ILC1s的增加或ILC1s向ILC3s的转分化,并且由细胞毒性ILC1s引起的NKp44激活诱导的丢失与干扰素(IFN)-γ表达增加相关释放溶菌颗粒。这些发现表明,上皮内NKp44-CD127-细胞毒性ILC1s可能导致CD的粘膜损伤。
更新日期:2020-01-27
中文翻译:

乳糜泻中小肠上皮内1型先天淋巴样细胞的表型转移与增强的细胞毒性潜力有关。
小肠(SI)上皮具有异质性淋巴细胞群,可在乳糜泻(CD)中介导粘膜损伤和修复。人类近端SI上皮内固有淋巴样细胞(ILC)的组成和作用,以及它们在CD中的变化,尚不十分清楚。我们报告十二指肠上皮内ILC主要由自然杀手(NK)p44 + CD127细胞毒性ILC1s和NKp44 CD127 +辅助ILC1s组成,而ILC3仅代表少数人群。在患有新诊断或活动性CD(ACD)和1型难治性CD(RCD I)的患者中,SI NKp44 + ILC的频率降低,坚持无麸质饮食并显示证据的NKp44 + ILC频率得以恢复。黏膜愈合。此外,SI NKp44-ILCs的频率在ACD和RCD I患者中增加,并与绒毛萎缩和上皮损害的严重程度相关,如通过血清脂肪酸结合蛋白2(FABP2)的水平评估。我们显示CD中的ILC改变代表细胞毒性ILC1的表型转变,而不是辅助ILC1s的增加或ILC1s向ILC3s的转分化,并且由细胞毒性ILC1s引起的NKp44激活诱导的丢失与干扰素(IFN)-γ表达增加相关释放溶菌颗粒。这些发现表明,上皮内NKp44-CD127-细胞毒性ILC1s可能导致CD的粘膜损伤。我们显示CD中的ILC改变代表细胞毒性ILC1的表型转变,而不是辅助ILC1s的增加或ILC1s向ILC3s的转分化,并且由细胞毒性ILC1s引起的NKp44激活诱导的丢失与干扰素(IFN)-γ表达增加相关释放溶菌颗粒。这些发现表明,上皮内NKp44-CD127-细胞毒性ILC1s可能导致CD的粘膜损伤。我们显示CD中的ILC改变代表细胞毒性ILC1的表型转变,而不是辅助ILC1s的增加或ILC1s向ILC3s的转分化,并且由细胞毒性ILC1s引起的NKp44激活诱导的丢失与干扰素(IFN)-γ表达增加相关释放溶菌颗粒。这些发现表明,上皮内NKp44-CD127-细胞毒性ILC1s可能导致CD的粘膜损伤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号