Protein & Peptide Letters ( IF 1.0 ) Pub Date : 2020-06-30 , DOI: 10.2174/0929866526666191204124245 Zhen Song 1 , Jin Liu 2 , Wen Yuan 1 , Ze Bai 1 , Nvwa Gao 1
|
Background: Recently, the small molecule that inhibits the human copper-trafficking proteins Atox1 and CCS was reported, which suggested that small molecule has an effect on the copper regulation system in the cell. The copper chaperones CopC is regarded as a redox switch and possess barrel structure, thus the interaction between CopC and small molecules could give helpful information to elucidate the copper regulation mechanism. In addition, porphyrins play an important role in the metabolism of living body. In the early-stage tumors, porphyrins were usually used to diagnosis. After the amphiphilic porphyrins were given by intravenous injection, serum albumins and serum proteins were the most usual carrier to transfer them. Then these molecules can accumulate in malignant tumours and contact with cancer cells. Obviously, in drug distribution and efficacy, investigation of the interaction between the porphyrins and protein is an important research area. Obviously, in drug distribution and efficacy, investigation of the interaction between the porphyrins and protein is an important research area.
Objective: In this article, our motivation is to establish a relation between Tetrakis (4- carboxylphenyl) porphyrin and CopC.
Methods: In this article, we propose a framework for achieving our aforementioned object. Firstly, FTIR spectra and CD were used to detect the structure changes of CopC. Secondly, the fluorescence spectroscopic and UV-Vis spectra were used to measure quenching mechanism, binding distance, binding site and binding distance. Using Tb 3+ as a probe to detect the interaction between CopC and TCPP. Finally, molecular docking methods was used to show the results more vivid.
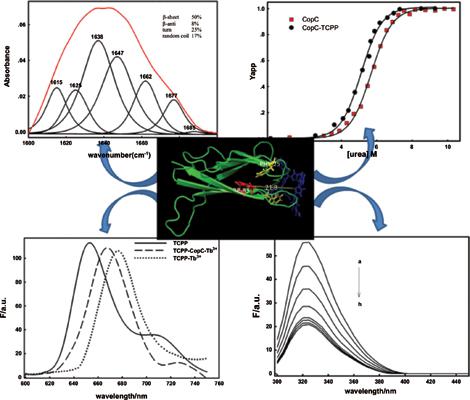
Results: Following the proposed framework, firstly, FTIR and CD results indicated that the CopC conformation was changed by TCPP. The β-sheet content was reduced and the random coil content was increased. Secondly, fluorescence spectra data indicated that the combination ratio of TCPPCopC was 1:1, and the inclusion constant is (5.88 ± 0.12) × 10 5 M -1 . In addition, Tb 3+ was used as a probe to detect the interaction between CopC and TCPP. The result further verified that CopC can interact with TCPP. The thermodynamic parameters of interaction between CopC and TCPP (ΔH, ΔS) indicated that the force between CopC and TCPP was mainly hydrophobic interaction. Finally, the distance between tryptophan in CopC and TCPP was calculated through forster energy transfer and molecular docking.
Conclusion: The results revealed that TCPP can form 1:1 complex with CopC, and the binding constant has been calculated to be (5.88 ± 0.12) × 10 5 M -1 . In addition, it was revealed that TCPP quench the fluorescence of CopC by the static quenching mechanism and the binding site n equals one. The formation of CopC-TCPP complex depended on the hydrophobic force and the distance between TCPP and tryptophan residue in CopC was 2.07 nm.
中文翻译:

用光谱和对接方法研究四(4-羧基苯基)卟啉与CopC之间的相互作用。
背景:最近,报道了抑制人类铜运输蛋白Atox1和CCS的小分子,这表明该小分子对细胞中的铜调节系统有影响。铜分子伴侣CopC被认为是一种氧化还原开关并具有桶状结构,因此CopC与小分子之间的相互作用可以为阐明铜的调控机理提供有益的信息。另外,卟啉在活体代谢中也起重要作用。在早期肿瘤中,卟啉通常用于诊断。静脉注射两亲性卟啉后,血清白蛋白和血清蛋白是最常见的载体。然后这些分子会积聚在恶性肿瘤中并与癌细胞接触。明显,在药物分布和功效方面,研究卟啉与蛋白质之间的相互作用是重要的研究领域。显然,在药物分布和功效方面,研究卟啉与蛋白质之间的相互作用是一个重要的研究领域。
目的:在本文中,我们的动机是建立四(4-羧基苯基)卟啉与CopC之间的关系。
方法:在本文中,我们提出了实现上述目标的框架。首先,用FTIR光谱和CD检测CopC的结构变化。其次,利用荧光光谱和紫外可见光谱测量猝灭机理,结合距离,结合部位和结合距离。使用Tb 3+作为探针来检测CopC和TCPP之间的相互作用。最后,使用分子对接方法显示结果更加生动。
结果:按照提出的框架,首先,FTIR和CD结果表明TCPP改变了CopC构象。β-片层含量减少,无规卷曲含量增加。其次,荧光光谱数据表明TCPPCopC的结合比为1:1,包合常数为(5.88±0.12)×10 5 M -1。此外,Tb 3+被用作探针来检测CopC和TCPP之间的相互作用。结果进一步证明CopC可以与TCPP交互。CopC和TCPP之间相互作用的热力学参数(ΔH,ΔS)表明CopC和TCPP之间的作用力主要是疏水相互作用。最后,通过Forster能量转移和分子对接计算了CopC中色氨酸与TCPP之间的距离。
结论:结果表明,TCPP与CopC可以形成1:1的复合物,结合常数经计算为(5.88±0.12)×10 5 M -1。另外,揭示了TCPP通过静态猝灭机制猝灭CopC的荧光,并且结合位点n等于1。CopC-TCPP复合物的形成取决于疏水力,CopC中TCPP与色氨酸残基之间的距离为2.07 nm。











































 京公网安备 11010802027423号
京公网安备 11010802027423号