Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia
JAMA ( IF 63.1 ) Pub Date : 2020-02-04 , DOI: 10.1001/jama.2019.22450 Myles Wolf 1 , Janet Rubin 2 , Maureen Achebe 3 , Michael J Econs 4 , Munro Peacock 4 , Erik A Imel 4 , Lars L Thomsen 5 , Thomas O Carpenter 6 , Thomas Weber 7 , Vincent Brandenburg 8 , Heinz Zoller 9
JAMA ( IF 63.1 ) Pub Date : 2020-02-04 , DOI: 10.1001/jama.2019.22450 Myles Wolf 1 , Janet Rubin 2 , Maureen Achebe 3 , Michael J Econs 4 , Munro Peacock 4 , Erik A Imel 4 , Lars L Thomsen 5 , Thomas O Carpenter 6 , Thomas Weber 7 , Vincent Brandenburg 8 , Heinz Zoller 9
Affiliation
|
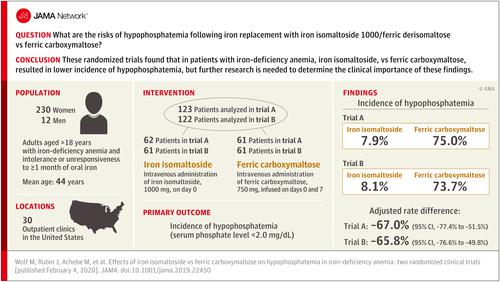
Importance
Intravenous iron enables rapid correction of iron-deficiency anemia, but certain formulations induce fibroblast growth factor 23-mediated hypophosphatemia. Objective
To compare risks of hypophosphatemia and effects on biomarkers of mineral and bone homeostasis of intravenous iron isomaltoside (now known as ferric derisomaltose) vs ferric carboxymaltose. Design, Setting, and Participants
Between October 2017 and June 2018, 245 patients aged 18 years and older with iron-deficiency anemia (hemoglobin level ≤11 g/dL; serum ferritin level ≤100 ng/mL) and intolerance or unresponsiveness to 1 month or more of oral iron were recruited from 30 outpatient clinic sites in the United States into 2 identically designed, open-label, randomized clinical trials. Patients with reduced kidney function were excluded. Serum phosphate and 12 additional biomarkers of mineral and bone homeostasis were measured on days 0, 1, 7, 8, 14, 21, and 35. The date of final follow-up was June 19, 2018, for trial A and May 29, 2018, for trial B. Interventions
Intravenous administration of iron isomaltoside, 1000 mg, on day 0 or ferric carboxymaltose, 750 mg, infused on days 0 and 7. Main Outcomes and Measures
The primary end point was the incidence of hypophosphatemia (serum phosphate level <2.0 mg/dL) between baseline and day 35. Results
In trial A, 123 patients were randomized (mean [SD] age, 45.1 [11.0] years; 95.9% women), including 62 to iron isomaltoside and 61 to ferric carboxymaltose; 95.1% completed the trial. In trial B, 122 patients were randomized (mean [SD] age, 42.6 [12.2] years; 94.1% women), including 61 to iron isomaltoside and 61 to ferric carboxymaltose; 93.4% completed the trial. The incidence of hypophosphatemia was significantly lower following iron isomaltoside vs ferric carboxymaltose (trial A: 7.9% vs 75.0% [adjusted rate difference, -67.0% {95% CI, -77.4% to -51.5%}], P < .001; trial B: 8.1% vs 73.7% [adjusted rate difference, -65.8% {95% CI, -76.6% to -49.8%}], P < .001). Beyond hypophosphatemia and increased parathyroid hormone, the most common adverse drug reactions (No./total No.) were nausea (iron isomaltoside: 1/125; ferric carboxymaltose: 8/117) and headache (iron isomaltoside: 4/125; ferric carboxymaltose: 5/117). Conclusions and Relevance
In 2 randomized trials of patients with iron-deficiency anemia who were intolerant of or unresponsive to oral iron, iron isomaltoside (now called ferric derisomaltose), compared with ferric carboxymaltose, resulted in lower incidence of hypophosphatemia over 35 days. However, further research is needed to determine the clinical importance of this difference. Trial Registration
ClinicalTrials.gov Identifiers: NCT03238911 and NCT03237065.
中文翻译:

异麦芽糖苷铁与羧基麦芽糖铁对缺铁性贫血低磷血症的影响
重要性 静脉补铁可以快速纠正缺铁性贫血,但某些制剂会诱发成纤维细胞生长因子 23 介导的低磷血症。目的比较静脉注射异麦芽糖苷铁(现称为去异麦芽糖铁)与羧基麦芽糖铁的低磷血症风险以及对矿物质和骨稳态生物标志物的影响。设计、背景和参与者 2017 年 10 月至 2018 年 6 月期间,245 名 18 岁及以上患有缺铁性贫血(血红蛋白水平≤11 g/dL;血清铁蛋白水平≤100 ng/mL)且对 1 个月不耐受或无反应的患者从美国 30 个门诊诊所招募了 1 名或更多口服铁剂,进行 2 项设计相同的开放标签随机临床试验。肾功能下降的患者被排除在外。在第 0、1、7、8、14、21 和 35 天测量血清磷酸盐以及 12 种矿物质和骨稳态的其他生物标志物。试验 A 的最终随访日期为 2018 年 6 月 19 日,试验 A 的最终随访日期为 5 月 29 日。 2018 年,试验 B。 干预措施 第 0 天静脉注射异麦芽糖苷铁 1000 mg,或第 0 天和第 7 天静脉注射羧基麦芽糖铁 750 mg。 主要结果和措施 主要终点是低磷血症的发生率(血清磷酸盐水平) <2.0 mg/dL)在基线和第 35 天之间。 结果 在试验 A 中,123 名患者被随机分配(平均 [SD] 年龄,45.1 [11.0] 岁;95.9% 为女性),其中 62 名患者接受异麦芽糖苷铁治疗,61 名患者接受羧基麦芽糖铁治疗;95.1% 完成了试验。在试验 B 中,122 名患者被随机分配(平均 [SD] 年龄,42.6 [12.2] 岁;94.1% 为女性),其中 61 名患者接受异麦芽糖苷铁治疗,61 名患者接受羧基麦芽糖铁治疗;93.4% 完成了试验。异麦芽糖苷铁与羧基麦芽糖铁相比,低磷血症的发生率显着降低(试验 A:7.9% vs 75.0% [调整后的比率差异,-67.0% {95% CI,-77.4% 至 -51.5%}],P < .001;试验 B:8.1% 与 73.7% [调整后的比率差异,-65.8% {95% CI,-76.6% 至 -49.8%}],P < .001)。除低磷血症和甲状旁腺激素增加外,最常见的药物不良反应(例数/总数)为恶心(异麦芽糖苷铁:1/125;羧基麦芽糖铁:8/117)和头痛(异麦芽糖苷铁:4/125;羧基麦芽糖铁) :5/117)。结论和相关性 在两项针对口服铁剂不耐受或无反应的缺铁性贫血患者的随机试验中,与羧基麦芽糖铁相比,异麦芽糖苷铁(现称为去异麦芽糖铁)可降低 35 天内低磷血症的发生率。然而,需要进一步的研究来确定这种差异的临床重要性。试验注册 ClinicalTrials.gov 标识符:NCT03238911 和 NCT03237065。
更新日期:2020-02-04
中文翻译:

异麦芽糖苷铁与羧基麦芽糖铁对缺铁性贫血低磷血症的影响
重要性 静脉补铁可以快速纠正缺铁性贫血,但某些制剂会诱发成纤维细胞生长因子 23 介导的低磷血症。目的比较静脉注射异麦芽糖苷铁(现称为去异麦芽糖铁)与羧基麦芽糖铁的低磷血症风险以及对矿物质和骨稳态生物标志物的影响。设计、背景和参与者 2017 年 10 月至 2018 年 6 月期间,245 名 18 岁及以上患有缺铁性贫血(血红蛋白水平≤11 g/dL;血清铁蛋白水平≤100 ng/mL)且对 1 个月不耐受或无反应的患者从美国 30 个门诊诊所招募了 1 名或更多口服铁剂,进行 2 项设计相同的开放标签随机临床试验。肾功能下降的患者被排除在外。在第 0、1、7、8、14、21 和 35 天测量血清磷酸盐以及 12 种矿物质和骨稳态的其他生物标志物。试验 A 的最终随访日期为 2018 年 6 月 19 日,试验 A 的最终随访日期为 5 月 29 日。 2018 年,试验 B。 干预措施 第 0 天静脉注射异麦芽糖苷铁 1000 mg,或第 0 天和第 7 天静脉注射羧基麦芽糖铁 750 mg。 主要结果和措施 主要终点是低磷血症的发生率(血清磷酸盐水平) <2.0 mg/dL)在基线和第 35 天之间。 结果 在试验 A 中,123 名患者被随机分配(平均 [SD] 年龄,45.1 [11.0] 岁;95.9% 为女性),其中 62 名患者接受异麦芽糖苷铁治疗,61 名患者接受羧基麦芽糖铁治疗;95.1% 完成了试验。在试验 B 中,122 名患者被随机分配(平均 [SD] 年龄,42.6 [12.2] 岁;94.1% 为女性),其中 61 名患者接受异麦芽糖苷铁治疗,61 名患者接受羧基麦芽糖铁治疗;93.4% 完成了试验。异麦芽糖苷铁与羧基麦芽糖铁相比,低磷血症的发生率显着降低(试验 A:7.9% vs 75.0% [调整后的比率差异,-67.0% {95% CI,-77.4% 至 -51.5%}],P < .001;试验 B:8.1% 与 73.7% [调整后的比率差异,-65.8% {95% CI,-76.6% 至 -49.8%}],P < .001)。除低磷血症和甲状旁腺激素增加外,最常见的药物不良反应(例数/总数)为恶心(异麦芽糖苷铁:1/125;羧基麦芽糖铁:8/117)和头痛(异麦芽糖苷铁:4/125;羧基麦芽糖铁) :5/117)。结论和相关性 在两项针对口服铁剂不耐受或无反应的缺铁性贫血患者的随机试验中,与羧基麦芽糖铁相比,异麦芽糖苷铁(现称为去异麦芽糖铁)可降低 35 天内低磷血症的发生率。然而,需要进一步的研究来确定这种差异的临床重要性。试验注册 ClinicalTrials.gov 标识符:NCT03238911 和 NCT03237065。











































 京公网安备 11010802027423号
京公网安备 11010802027423号