当前位置:
X-MOL 学术
›
J. Chromatogr. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Method development and validation of dissolution testing for nicotine release from smokeless tobacco products using flow-through cell apparatus and UPLC-PDA.
Journal of Chromatography B ( IF 2.8 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.jchromb.2020.122012 John H Miller 1 , Tim Danielson 1 , Yezdi B Pithawalla 1 , Anthony P Brown 1 , Celeste Wilkinson 1 , Karl Wagner 1 , Fadi Aldeek 1
Journal of Chromatography B ( IF 2.8 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.jchromb.2020.122012 John H Miller 1 , Tim Danielson 1 , Yezdi B Pithawalla 1 , Anthony P Brown 1 , Celeste Wilkinson 1 , Karl Wagner 1 , Fadi Aldeek 1
Affiliation
|
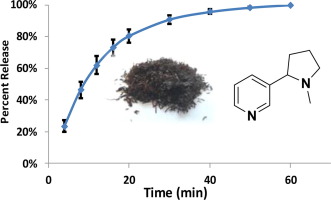
Developing dissolution testing methods to measure the nicotine release profiles from smokeless tobacco products is valuable for product assessment and product-to-product comparisons. In this work, we developed a robust dissolution method to study the in vitro release of nicotine from smokeless tobacco products using the U.S. Pharmacopeia flow-through cell dissolution apparatus 4 (USP-4). We further developed and validated a sensitive Ultra Performance Liquid Chromatography coupled to Photodiode Array detector (UPLC-PDA) method for the accurate quantitation of the released nicotine into artificial saliva, which is our selected dissolution medium. We have successfully shown the applicability of the validated method by investigating the release profiles of nicotine from various commercial and CORESTA reference smokeless tobacco products [CRP 1.1 (Swedish-style snus pouch), CRP 2.1 (American-style loose moist snuff), CRP 4 (loose-leaf chewing tobacco) and CRP 4.1 (chopped loose-leaf chewing tobacco)]. Nicotine release profiles were analyzed by calculating the difference factor (f1) and similarity factor (f2) by adopting a methodology referenced in the Guidance for Industry from FDA's Center for Drug Evaluation and Research (CDER) and by fitting the release profile curves using a first order kinetic model. Nicotine release was found to be dependent on the form and cut of the smokeless tobacco products, with a slower release observed for snus and loose-leaf, compared to chopped and loose moist snuff smokeless tobacco. This dissolution methodology can be extended to measure and compare release of other constituents from smokeless tobacco products and has the potential for method standardization.
中文翻译:

使用流通池设备和UPLC-PDA对无烟烟草产品中尼古丁释放进行溶出度测试的方法开发和验证。
开发用于测量无烟烟草产品中尼古丁释放曲线的溶出度测试方法对于产品评估和产品之间的比较非常有价值。在这项工作中,我们开发了一种强大的溶解方法,以研究使用美国药典流通型细胞溶解仪4(USP-4)从无烟烟草制品中体外释放尼古丁的方法。我们进一步开发并验证了灵敏的超高效液相色谱与光电二极管阵列检测器(UPLC-PDA)结合使用的方法,可准确定量释放到人工唾液中的尼古丁,这是我们选择的溶解介质。通过调查各种商业和CORESTA参考无烟烟草产品[CRP 1]尼古丁的释放曲线,我们已成功证明了验证方法的适用性。1(瑞典式鼻烟袋),CRP 2.1(美式散装湿鼻烟),CRP 4(散叶嚼烟)和CRP 4.1(切碎的散叶嚼烟)]。通过采用FDA药品评估与研究中心(CDER)的《工业指南》中引用的方法,通过计算差异因子(f1)和相似因子(f2)并通过使用第一阶动力学模型。发现尼古丁的释放取决于无烟烟草产品的形式和切面,与切碎和湿润的鼻烟无烟烟草相比,鼻烟和活页烟的释放较慢。
更新日期:2020-02-04
中文翻译:

使用流通池设备和UPLC-PDA对无烟烟草产品中尼古丁释放进行溶出度测试的方法开发和验证。
开发用于测量无烟烟草产品中尼古丁释放曲线的溶出度测试方法对于产品评估和产品之间的比较非常有价值。在这项工作中,我们开发了一种强大的溶解方法,以研究使用美国药典流通型细胞溶解仪4(USP-4)从无烟烟草制品中体外释放尼古丁的方法。我们进一步开发并验证了灵敏的超高效液相色谱与光电二极管阵列检测器(UPLC-PDA)结合使用的方法,可准确定量释放到人工唾液中的尼古丁,这是我们选择的溶解介质。通过调查各种商业和CORESTA参考无烟烟草产品[CRP 1]尼古丁的释放曲线,我们已成功证明了验证方法的适用性。1(瑞典式鼻烟袋),CRP 2.1(美式散装湿鼻烟),CRP 4(散叶嚼烟)和CRP 4.1(切碎的散叶嚼烟)]。通过采用FDA药品评估与研究中心(CDER)的《工业指南》中引用的方法,通过计算差异因子(f1)和相似因子(f2)并通过使用第一阶动力学模型。发现尼古丁的释放取决于无烟烟草产品的形式和切面,与切碎和湿润的鼻烟无烟烟草相比,鼻烟和活页烟的释放较慢。











































 京公网安备 11010802027423号
京公网安备 11010802027423号