当前位置:
X-MOL 学术
›
Nat. Protoc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid online buffer exchange for screening of proteins, protein complexes and cell lysates by native mass spectrometry.
Nature Protocols ( IF 13.1 ) Pub Date : 2020-01-31 , DOI: 10.1038/s41596-019-0281-0 Zachary L VanAernum 1, 2 , Florian Busch 1, 2 , Benjamin J Jones 1, 2 , Mengxuan Jia 1, 2 , Zibo Chen 3, 4, 5 , Scott E Boyken 3, 4, 6 , Aniruddha Sahasrabuddhe 1, 7 , David Baker 3, 4, 8 , Vicki H Wysocki 1, 2
Nature Protocols ( IF 13.1 ) Pub Date : 2020-01-31 , DOI: 10.1038/s41596-019-0281-0 Zachary L VanAernum 1, 2 , Florian Busch 1, 2 , Benjamin J Jones 1, 2 , Mengxuan Jia 1, 2 , Zibo Chen 3, 4, 5 , Scott E Boyken 3, 4, 6 , Aniruddha Sahasrabuddhe 1, 7 , David Baker 3, 4, 8 , Vicki H Wysocki 1, 2
Affiliation
|
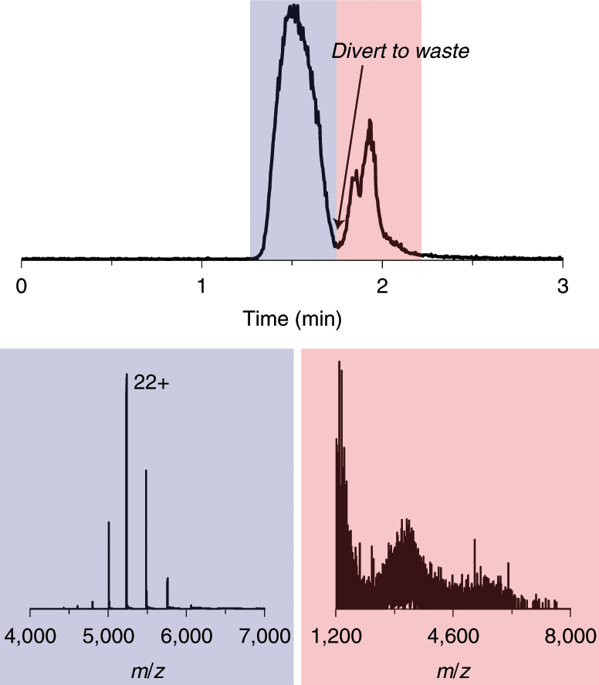
It is important to assess the identity and purity of proteins and protein complexes during and after protein purification to ensure that samples are of sufficient quality for further biochemical and structural characterization, as well as for use in consumer products, chemical processes and therapeutics. Native mass spectrometry (nMS) has become an important tool in protein analysis due to its ability to retain non-covalent interactions during measurements, making it possible to obtain protein structural information with high sensitivity and at high speed. Interferences from the presence of non-volatiles are typically alleviated by offline buffer exchange, which is time-consuming and difficult to automate. We provide a protocol for rapid online buffer exchange (OBE) nMS to directly screen structural features of pre-purified proteins, protein complexes or clarified cell lysates. In the liquid chromatography coupled to mass spectrometry (LC-MS) approach described in this protocol, samples in MS-incompatible conditions are injected onto a short size-exclusion chromatography column. Proteins and protein complexes are separated from small molecule non-volatile buffer components using an aqueous, non-denaturing mobile phase. Eluted proteins and protein complexes are detected by the mass spectrometer after electrospray ionization. Mass spectra can inform regarding protein sample purity and oligomerization, and additional tandem mass spectra can help to further obtain information on protein complex subunits. Information obtained by OBE nMS can be used for fast (<5 min) quality control and can further guide protein expression and purification optimization.
中文翻译:

快速在线缓冲液交换,用于通过天然质谱法筛选蛋白质、蛋白质复合物和细胞裂解物。
在蛋白质纯化期间和之后评估蛋白质和蛋白质复合物的身份和纯度非常重要,以确保样品具有足够的质量以进行进一步的生化和结构表征,以及用于消费品、化学过程和治疗。天然质谱(nMS)因其在测量过程中保留非共价相互作用的能力而成为蛋白质分析的重要工具,使得高灵敏度和高速获得蛋白质结构信息成为可能。非易失性物质存在的干扰通常通过离线缓冲区交换来减轻,这种交换既耗时又难以实现自动化。我们提供快速在线缓冲液交换 (OBE) nMS 方案,以直接筛选预纯化蛋白质、蛋白质复合物或澄清细胞裂解物的结构特征。在本协议中描述的液相色谱耦合质谱 (LC-MS) 方法中,MS 不兼容条件下的样品被注射到短尺寸排阻色谱柱上。使用水性非变性流动相将蛋白质和蛋白质复合物与小分子非挥发性缓冲液成分分离。电喷雾电离后,通过质谱仪检测洗脱的蛋白质和蛋白质复合物。质谱可以提供有关蛋白质样品纯度和寡聚化的信息,而额外的串联质谱可以帮助进一步获得有关蛋白质复合物亚基的信息。 OBE nMS 获得的信息可用于快速(<5 分钟)质量控制,并可进一步指导蛋白质表达和纯化优化。
更新日期:2020-01-31
中文翻译:

快速在线缓冲液交换,用于通过天然质谱法筛选蛋白质、蛋白质复合物和细胞裂解物。
在蛋白质纯化期间和之后评估蛋白质和蛋白质复合物的身份和纯度非常重要,以确保样品具有足够的质量以进行进一步的生化和结构表征,以及用于消费品、化学过程和治疗。天然质谱(nMS)因其在测量过程中保留非共价相互作用的能力而成为蛋白质分析的重要工具,使得高灵敏度和高速获得蛋白质结构信息成为可能。非易失性物质存在的干扰通常通过离线缓冲区交换来减轻,这种交换既耗时又难以实现自动化。我们提供快速在线缓冲液交换 (OBE) nMS 方案,以直接筛选预纯化蛋白质、蛋白质复合物或澄清细胞裂解物的结构特征。在本协议中描述的液相色谱耦合质谱 (LC-MS) 方法中,MS 不兼容条件下的样品被注射到短尺寸排阻色谱柱上。使用水性非变性流动相将蛋白质和蛋白质复合物与小分子非挥发性缓冲液成分分离。电喷雾电离后,通过质谱仪检测洗脱的蛋白质和蛋白质复合物。质谱可以提供有关蛋白质样品纯度和寡聚化的信息,而额外的串联质谱可以帮助进一步获得有关蛋白质复合物亚基的信息。 OBE nMS 获得的信息可用于快速(<5 分钟)质量控制,并可进一步指导蛋白质表达和纯化优化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号