Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.mcat.2019.110739 Jan M. Klenk , Julia Ertl , Lea Rapp , Max-Philipp Fischer , Bernhard Hauer
|
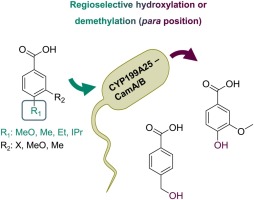
Metabolically diverse organisms can serve as valuable source for Cytochrome P450 monooxygenases (CYPs), extending the repertoire of biocatalysts for C–H bond oxyfunctionalization. Here, we report on the cloning, expression and characterization of two new P450 s from the gram-positive papaverine degrading Arthrobacter sp. The activity of one of these P450 s, CYP199A25, could be reconstituted enabling its detailed biochemical characterization. CYP199A25 has shown to be a regioselective benzoic acid hydroxylase which demethylates or hydroxylates only in para position using the non-physiological redox partners CamA and CamB from P. putida. In accordance with the previously published CYP199A members, the defined enzyme architecture determines the high regioselectivity. While the construction of a fusion protein gave low activity in vitro and in vivo, a whole-cell system comprising of the compatible pBAD18/33 plasmids produced 1.3 g L−1 of the final product 4-hydroxybenzoic acid corresponding to a total conversion of 94 %. CYP199A25 represents a highly selective and efficient biocatalyst complementing the interesting CYP199A subfamily for future applications.
中文翻译:

表达和苯甲酸羟化酶CYP199A25的表征节藻。
代谢多样的生物可以作为细胞色素P450单加氧酶(CYP)的宝贵来源,扩展了C–H键氧官能化的生物催化剂的种类。在这里,我们报道了革兰氏阳性罂粟碱降解节杆菌的两个新P450的克隆,表达和鉴定。这些P450之一CYP199A25的活性可以重建,从而能够对其进行详细的生化表征。CYP199A25是一种区域选择性的苯甲酸羟化酶,仅使用恶臭假单胞菌的非生理氧化还原伙伴CamA和CamB在对位脱甲基或羟化。根据以前发布的CYP199A成员,定义的酶结构决定了高区域选择性。尽管融合蛋白的构建在体外和体内均具有较低的活性,但由兼容的pBAD18 / 33质粒组成的全细胞系统可产生1.3 g L -1的最终产物4-羟基苯甲酸,相当于94的总转化率%。CYP199A25代表了一种高度选择性和高效的生物催化剂,是对将来有趣的CYP199A亚家族的补充。











































 京公网安备 11010802027423号
京公网安备 11010802027423号