当前位置:
X-MOL 学术
›
PLOS Genet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of yeast Rad51 and Rad52 relieves Rad52-mediated inhibition of de novo telomere addition.
PLOS Genetics ( IF 4.0 ) Pub Date : 2020-02-03 , DOI: 10.1371/journal.pgen.1008608 Esther A Epum 1 , Michael J Mohan 1 , Nicholas P Ruppe 1 , Katherine L Friedman 1
PLOS Genetics ( IF 4.0 ) Pub Date : 2020-02-03 , DOI: 10.1371/journal.pgen.1008608 Esther A Epum 1 , Michael J Mohan 1 , Nicholas P Ruppe 1 , Katherine L Friedman 1
Affiliation
|
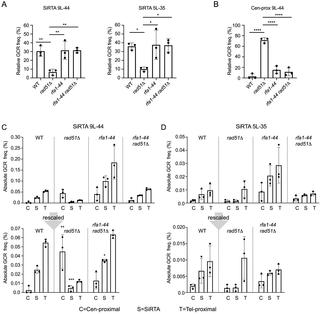
DNA double-strand breaks (DSBs) are toxic forms of DNA damage that must be repaired to maintain genome integrity. Telomerase can act upon a DSB to create a de novo telomere, a process that interferes with normal repair and creates terminal deletions. We previously identified sequences in Saccharomyces cerevisiae (SiRTAs; Sites of Repair-associated Telomere Addition) that undergo unusually high frequencies of de novo telomere addition, even when the original chromosome break is several kilobases distal to the eventual site of telomerase action. Association of the single-stranded telomere binding protein Cdc13 with a SiRTA is required to stimulate de novo telomere addition. Because extensive resection must occur prior to Cdc13 binding, we utilized these sites to monitor the effect of proteins involved in homologous recombination. We find that telomere addition is significantly reduced in the absence of the Rad51 recombinase, while loss of Rad52, required for Rad51 nucleoprotein filament formation, has no effect. Deletion of RAD52 suppresses the defect of the rad51Δ strain, suggesting that Rad52 inhibits de novo telomere addition in the absence of Rad51. The ability of Rad51 to counteract this effect of Rad52 does not require DNA binding by Rad51, but does require interaction between the two proteins, while the inhibitory effect of Rad52 depends on its interaction with Replication Protein A (RPA). Intriguingly, the genetic interactions we report between RAD51 and RAD52 are similar to those previously observed in the context of checkpoint adaptation. Forced recruitment of Cdc13 fully restores telomere addition in the absence of Rad51, suggesting that Rad52, through its interaction with RPA-coated single-stranded DNA, inhibits the ability of Cdc13 to bind and stimulate telomere addition. Loss of the Rad51-Rad52 interaction also stimulates a subset of Rad52-dependent microhomology-mediated repair (MHMR) events, consistent with the known ability of Rad51 to prevent single-strand annealing.
中文翻译:

酵母Rad51和Rad52的相互作用缓解了Rad52介导的从头端粒添加的抑制作用。
DNA双链断裂(DSB)是DNA损伤的有毒形式,必须对其进行修复以保持基因组完整性。端粒酶可以作用于DSB来产生从头端粒,该过程会干扰正常修复并产生末端缺失。我们以前在酿酒酵母(SiRTAs;修复相关的端粒加成位点)中鉴定了序列,这些序列经历了从头端粒加成的异常高频率,即使原始染色体断裂距离端粒酶作用的最终位点几千个碱基也是如此。需要单链端粒结合蛋白Cdc13与SiRTA结合以刺激从头端粒的添加。因为必须在Cdc13结合之前进行广泛的切除,所以我们利用这些位点来监测参与同源重组的蛋白质的作用。我们发现,在不存在Rad51重组酶的情况下,端粒的添加显着减少,而Rad51核蛋白丝形成所需的Rad52丢失则没有影响。RAD52的缺失抑制了rad51Δ菌株的缺陷,表明Rad52在不存在Rad51的情况下抑制从头端粒的添加。Rad51抵消Rad52的这种作用的能力不需要Rad51与DNA结合,而是需要两种蛋白质之间的相互作用,而Rad52的抑制作用取决于它与复制蛋白A(RPA)的相互作用。有趣的是,我们报道的RAD51和RAD52之间的遗传相互作用与先前在检查点适应中观察到的相似。在不使用Rad51的情况下,强制招募Cdc13可以完全恢复端粒的添加,这表明Rad52,通过与RPA包被的单链DNA的相互作用,抑制了Cdc13结合并刺激端粒添加的能力。Rad51-Rad52相互作用的丧失也刺激了Rad52依赖的微同源介导的修复(MHMR)事件的子集,这与Rad51防止单链退火的已知能力一致。
更新日期:2020-03-05
中文翻译:

酵母Rad51和Rad52的相互作用缓解了Rad52介导的从头端粒添加的抑制作用。
DNA双链断裂(DSB)是DNA损伤的有毒形式,必须对其进行修复以保持基因组完整性。端粒酶可以作用于DSB来产生从头端粒,该过程会干扰正常修复并产生末端缺失。我们以前在酿酒酵母(SiRTAs;修复相关的端粒加成位点)中鉴定了序列,这些序列经历了从头端粒加成的异常高频率,即使原始染色体断裂距离端粒酶作用的最终位点几千个碱基也是如此。需要单链端粒结合蛋白Cdc13与SiRTA结合以刺激从头端粒的添加。因为必须在Cdc13结合之前进行广泛的切除,所以我们利用这些位点来监测参与同源重组的蛋白质的作用。我们发现,在不存在Rad51重组酶的情况下,端粒的添加显着减少,而Rad51核蛋白丝形成所需的Rad52丢失则没有影响。RAD52的缺失抑制了rad51Δ菌株的缺陷,表明Rad52在不存在Rad51的情况下抑制从头端粒的添加。Rad51抵消Rad52的这种作用的能力不需要Rad51与DNA结合,而是需要两种蛋白质之间的相互作用,而Rad52的抑制作用取决于它与复制蛋白A(RPA)的相互作用。有趣的是,我们报道的RAD51和RAD52之间的遗传相互作用与先前在检查点适应中观察到的相似。在不使用Rad51的情况下,强制招募Cdc13可以完全恢复端粒的添加,这表明Rad52,通过与RPA包被的单链DNA的相互作用,抑制了Cdc13结合并刺激端粒添加的能力。Rad51-Rad52相互作用的丧失也刺激了Rad52依赖的微同源介导的修复(MHMR)事件的子集,这与Rad51防止单链退火的已知能力一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号