当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amino Alcohol Acrylonitriles as Activators of the Aryl Hydrocarbon Receptor Pathway: An Unexpected MTT Phenotypic Screening Outcome.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-02-03 , DOI: 10.1002/cmdc.201900643 Jennifer R Baker 1 , Cecilia C Russell 1 , Jayne Gilbert 2 , Jennette A Sakoff 2 , Adam McCluskey 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-02-03 , DOI: 10.1002/cmdc.201900643 Jennifer R Baker 1 , Cecilia C Russell 1 , Jayne Gilbert 2 , Jennette A Sakoff 2 , Adam McCluskey 1
Affiliation
|
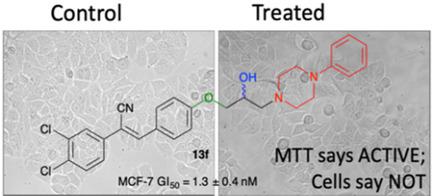
Lead (Z)-N-(4-(2-cyano-2-(3,4-dichlorophenyl)vinyl)phenyl)acetamide, 1 showed MCF-7 GI50 =30 nM and 400-fold selective c.f. MCF10A (normal breast tissue). Acetamide moiety modification (13 a-g) to introduce additional hydrophobicity was favoured with MCF-7 breast cancer cell activity enhanced at 1.3 nM. Other analogues were potent against the HT29 colon cancer cell line at 23 nM. Textbook SAR data was observed in the MCF-7 cell line, in an MTT assay, via the ortho (17 a), meta (17 b) and para (13 f). The amino alcohol -OH moiety was pivotal, but no stereochemical preference noted. But, these data did not fit our homology modelling expectations. Aberrant MTT ((3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) screening results and metabolic interference confirmed by sulforhodamine B (SRB) screening. Interfering analogues resulted in 120 and 80-fold CYP1A1 and CYP1A2 amplification, with no upregulation of SULT1A1. This is consistent with activation of the AhR pathway. Piperidine per-deuteration reduced metabolic inactivation. 3-OH / 4-OH piperidine analogues showed differential MTT and SRB activity supporting MTT assay metabolic inactivation. Data supports piperidine 3-OH, but not the 4-OH, as a CYP substrate. This family of β-amino alcohol substituted 3,4-dichlorophenylacetonitriles show broad activity modulated via the AhR pathway. By SRB analysis the most potent analogue was 23 b, (Z)-3-(4-(3-(4-phenylpiperidin-1-yl)-2-hydroxypropoxy)phenyl)-2-(3,4-dichlorophenyl)-acrylonitrile.
中文翻译:

氨基醇丙烯腈作为芳烃受体途径的活化剂:意外的MTT表型筛选结果。
铅(Z)-N-(4-(2-氰基-2-(3,4-二氯苯基)乙烯基)苯基)乙酰胺,1显示MCF-7 GI50 = 30 nM和400倍选择性cf MCF10A(正常乳房组织)。乙酰胺部分修饰(13 ag)引入了更多的疏水性,使MCF-7乳腺癌细胞的活性增强了1.3 nM,这是有利的。其他类似物对HT29结肠癌细胞系的作用为23 nM。在MTT分析中,通过邻位(17 a),间位(17 b)和对位(13 f)在MCF-7细胞系中观察到教科书SAR数据。氨基醇-OH部分是关键的,但没有注意到立体化学偏好。但是,这些数据不符合我们的同源建模预期。异常MTT((3- [4,5-二甲基噻唑-2-基] -2,5-二苯基四唑鎓溴化物)筛选结果和代谢干扰已通过磺基罗丹明B(SRB)筛选确认。干扰类似物导致CYP1A1和CYP1A2扩增120倍和80倍,而SULT1A1没有上调。这与AhR途径的激活是一致的。哌啶每次氘化可减少代谢失活。3-OH / 4-OH哌啶类似物显示出不同的MTT和SRB活性,支持MTT分析代谢失活。数据支持哌啶3-OH,但不支持4-OH作为CYP底物。β-氨基醇取代的3,4-二氯苯基乙腈家族显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。哌啶每次氘化可减少代谢失活。3-OH / 4-OH哌啶类似物显示出不同的MTT和SRB活性,支持MTT分析代谢失活。数据支持哌啶3-OH,但不支持4-OH作为CYP底物。β-氨基醇取代的3,4-二氯苯基乙腈家族显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。哌啶每次氘化可减少代谢失活。3-OH / 4-OH哌啶类似物显示出不同的MTT和SRB活性,支持MTT分析代谢失活。数据支持哌啶3-OH,但不支持4-OH作为CYP底物。β-氨基醇取代的3,4-二氯苯基乙腈家族显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。4-二氯苯基乙腈显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。4-二氯苯基乙腈显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。
更新日期:2020-02-28
中文翻译:

氨基醇丙烯腈作为芳烃受体途径的活化剂:意外的MTT表型筛选结果。
铅(Z)-N-(4-(2-氰基-2-(3,4-二氯苯基)乙烯基)苯基)乙酰胺,1显示MCF-7 GI50 = 30 nM和400倍选择性cf MCF10A(正常乳房组织)。乙酰胺部分修饰(13 ag)引入了更多的疏水性,使MCF-7乳腺癌细胞的活性增强了1.3 nM,这是有利的。其他类似物对HT29结肠癌细胞系的作用为23 nM。在MTT分析中,通过邻位(17 a),间位(17 b)和对位(13 f)在MCF-7细胞系中观察到教科书SAR数据。氨基醇-OH部分是关键的,但没有注意到立体化学偏好。但是,这些数据不符合我们的同源建模预期。异常MTT((3- [4,5-二甲基噻唑-2-基] -2,5-二苯基四唑鎓溴化物)筛选结果和代谢干扰已通过磺基罗丹明B(SRB)筛选确认。干扰类似物导致CYP1A1和CYP1A2扩增120倍和80倍,而SULT1A1没有上调。这与AhR途径的激活是一致的。哌啶每次氘化可减少代谢失活。3-OH / 4-OH哌啶类似物显示出不同的MTT和SRB活性,支持MTT分析代谢失活。数据支持哌啶3-OH,但不支持4-OH作为CYP底物。β-氨基醇取代的3,4-二氯苯基乙腈家族显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。哌啶每次氘化可减少代谢失活。3-OH / 4-OH哌啶类似物显示出不同的MTT和SRB活性,支持MTT分析代谢失活。数据支持哌啶3-OH,但不支持4-OH作为CYP底物。β-氨基醇取代的3,4-二氯苯基乙腈家族显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。哌啶每次氘化可减少代谢失活。3-OH / 4-OH哌啶类似物显示出不同的MTT和SRB活性,支持MTT分析代谢失活。数据支持哌啶3-OH,但不支持4-OH作为CYP底物。β-氨基醇取代的3,4-二氯苯基乙腈家族显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。4-二氯苯基乙腈显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。4-二氯苯基乙腈显示出通过AhR途径调节的广泛活性。通过SRB分析,最有效的类似物是23 b,(Z)-3-(4-(3-(4-苯基哌啶-1-基)-2-羟基丙氧基)苯基)-2-(3,4-二氯苯基)-丙烯腈。











































 京公网安备 11010802027423号
京公网安备 11010802027423号