当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of carbon dioxide (CO2) in aqueous solution of 3-(dimethylamino)-1-propylamine (DMAPA)
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.fluid.2020.112506 Devjyoti Nath , Amr Henni
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.fluid.2020.112506 Devjyoti Nath , Amr Henni
|
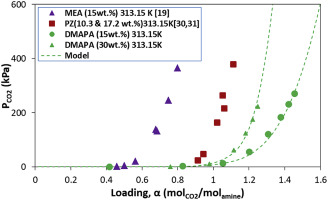
Abstract The main objective of this study is to investigate the efficiency of 3-(Dimethylamino)-1-propylamine (DMAPA) as a solvent for carbon dioxide (CO2) capture. The solubility of CO2 in aqueous solutions of DMAPA was measured at (313.15 and 333.15) K and at a partial pressure of CO2 up to 275 kPa using the pressure-decay method. Measurements were performed at two concentrations of DMAPA (15 wt % and 30 wt %). DMAPA exhibited high CO2 loading when compared with other benchmark amines for CO2 absorption technology such as mono-ethanolamine (MEA) and piperazine (PZ). In this study, the highest CO2 loading recorded was 1.46 at 313.15 K at a CO2 partial pressure of 271 kPa. The experimental solubility data were correlated with the electrolyte-NRTL model, and the Redlich-Kwong (RK) equation of state was used for the estimation of the vapor phase fugacity coefficients at equilibrium. Excess enthalpy (HE) for DMAPA-water binary system was measured using a C80 flow calorimeter at (313.15 and 333.15) K. Binary NRTL parameters for DMAPA/H2O were regressed from the excess enthalpy (HE) data of the DMAPA-water system; and some other parameters such as the molecule-ion pair parameters, equilibrium constants, etc. were regressed using vapor-liquid equilibrium (VLE) data. Finally, the Gibbs-Helmholtz equation was used to predict the heat of absorption of CO2 solubility in the amine solution based on solubility data.
中文翻译:

二氧化碳 (CO2) 在 3-(二甲氨基)-1-丙胺 (DMAPA) 水溶液中的溶解度
摘要 本研究的主要目的是研究 3-(二甲氨基)-1-丙胺 (DMAPA) 作为二氧化碳 (CO2) 捕获溶剂的效率。CO2 在 DMAPA 水溶液中的溶解度是在 (313.15 和 333.15) K 和 CO2 分压高达 275 kPa 下使用压力衰减法测量的。测量在两种浓度的 DMAPA(15 重量%和 30 重量%)下进行。与其他用于 CO2 吸收技术的基准胺(如单乙醇胺 (MEA) 和哌嗪 (PZ))相比,DMAPA 表现出较高的 CO2 负载量。在这项研究中,记录的最高 CO2 负荷为 1.46,在 313.15 K,CO2 分压为 271 kPa。实验溶解度数据与电解质-NRTL模型相关,Redlich-Kwong (RK) 状态方程用于估计平衡时的气相逸度系数。DMAPA-水二元系统的过量焓 (HE) 使用 C80 流量热量计在 (313.15 和 333.15) K 处测量。DMAPA/H2O 的二元 NRTL 参数从 DMAPA-水系统的过量焓 (HE) 数据回归;使用汽液平衡 (VLE) 数据对分子离子对参数、平衡常数等其他一些参数进行回归。最后,基于溶解度数据,使用 Gibbs-Helmholtz 方程来预测 CO2 在胺溶液中溶解度的吸收热。DMAPA/H2O 的二元 NRTL 参数从 DMAPA-水系统的过量焓 (HE) 数据回归;使用汽液平衡 (VLE) 数据对分子离子对参数、平衡常数等其他一些参数进行回归。最后,基于溶解度数据,使用 Gibbs-Helmholtz 方程来预测 CO2 在胺溶液中溶解度的吸收热。DMAPA/H2O 的二元 NRTL 参数从 DMAPA-水系统的过量焓 (HE) 数据回归;使用汽液平衡 (VLE) 数据对分子离子对参数、平衡常数等其他一些参数进行回归。最后,基于溶解度数据,使用 Gibbs-Helmholtz 方程来预测 CO2 在胺溶液中溶解度的吸收热。
更新日期:2020-05-01
中文翻译:

二氧化碳 (CO2) 在 3-(二甲氨基)-1-丙胺 (DMAPA) 水溶液中的溶解度
摘要 本研究的主要目的是研究 3-(二甲氨基)-1-丙胺 (DMAPA) 作为二氧化碳 (CO2) 捕获溶剂的效率。CO2 在 DMAPA 水溶液中的溶解度是在 (313.15 和 333.15) K 和 CO2 分压高达 275 kPa 下使用压力衰减法测量的。测量在两种浓度的 DMAPA(15 重量%和 30 重量%)下进行。与其他用于 CO2 吸收技术的基准胺(如单乙醇胺 (MEA) 和哌嗪 (PZ))相比,DMAPA 表现出较高的 CO2 负载量。在这项研究中,记录的最高 CO2 负荷为 1.46,在 313.15 K,CO2 分压为 271 kPa。实验溶解度数据与电解质-NRTL模型相关,Redlich-Kwong (RK) 状态方程用于估计平衡时的气相逸度系数。DMAPA-水二元系统的过量焓 (HE) 使用 C80 流量热量计在 (313.15 和 333.15) K 处测量。DMAPA/H2O 的二元 NRTL 参数从 DMAPA-水系统的过量焓 (HE) 数据回归;使用汽液平衡 (VLE) 数据对分子离子对参数、平衡常数等其他一些参数进行回归。最后,基于溶解度数据,使用 Gibbs-Helmholtz 方程来预测 CO2 在胺溶液中溶解度的吸收热。DMAPA/H2O 的二元 NRTL 参数从 DMAPA-水系统的过量焓 (HE) 数据回归;使用汽液平衡 (VLE) 数据对分子离子对参数、平衡常数等其他一些参数进行回归。最后,基于溶解度数据,使用 Gibbs-Helmholtz 方程来预测 CO2 在胺溶液中溶解度的吸收热。DMAPA/H2O 的二元 NRTL 参数从 DMAPA-水系统的过量焓 (HE) 数据回归;使用汽液平衡 (VLE) 数据对分子离子对参数、平衡常数等其他一些参数进行回归。最后,基于溶解度数据,使用 Gibbs-Helmholtz 方程来预测 CO2 在胺溶液中溶解度的吸收热。











































 京公网安备 11010802027423号
京公网安备 11010802027423号