Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.jphotochem.2020.112436 Viona Wongso , Hui Khee Chung , Nonni Soraya Sambudi , Suriati Sufian , Bawadi Abdullah , Muhammad Dzul Hakim Wirzal , Wei Lun Ang
|
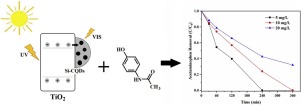
The presence of pharmaceutical compound (i.e., acetaminophen) in aquatic environment has been declared as environmental issue since researchers found that it has potential risk to human health. Photocatalytic process as a promising method for waste degradation commonly employs titanium dioxide, TiO2. However, TiO2 has narrow light absorption and rapid charge recombination resulting in ineffective photocatalytic activity. In this study, silica – carbon quantum dots (Si-CQDs) from rice husk are decorated into TiO2 matrix through facile mixing approach to minimize the limitations of TiO2. Preliminary studies regarding TiO2 transformation and Si-CQDs incorporation in various amount were systematically investigated. It is observed that 1 wt% is the optimum amount of Si-CQDs in composite in order to maximize the photocatalytic ability of TiO2. Under sunlight irradiation, 1 wt% Si-CQDs/TiO2 composite is able to completely degrade 5 mg/L of acetaminophen within 240 min (33.3 % faster than pure TiO2). The excellent performance of the composite is attributed to synergistic effect of Si-CQDs addition on TiO2 surface, which acted as photo sensitizer and electron trapper. Si-CQDs extend light absorption of TiO2 by reducing band gap energy from 3.20 to 3.12 eV, as confirmed by UV–vis Diffuse Reflectance Spectroscopy (DRS) spectra. Photoluminescence (PL) spectra and N2 sorption isotherm reveal that Si-CQDs addition prolongs the lifetime of charge separation and improves surface area (17 % larger than TiO2), respectively. The composite of Si-CQDs/TiO2 also demonstrates good stability which is beneficial for pharmaceutical waste removal in the future.
中文翻译:

二氧化硅-碳量子点将二氧化钛装饰为阳光驱动的光催化剂,以减少水生环境中的对乙酰氨基酚
自从研究人员发现在水生环境中存在药用化合物(即对乙酰氨基酚)以来,就已经将其宣布为环境问题,因为研究人员发现该化合物对人体健康具有潜在风险。作为一种有希望的废物降解方法,光催化工艺通常使用二氧化钛TiO 2。然而,TiO 2具有窄的光吸收和快速的电荷复合,导致无效的光催化活性。在这项研究中,通过简便的混合方法将稻壳中的二氧化硅-碳量子点(Si-CQDs)装饰到TiO 2基质中,以最小化TiO 2的限制。TiO 2的初步研究系统地研究了各种形式的转变和Si-CQDs的掺入。观察到为了使TiO 2的光催化能力最大化,在复合物中Si-CQD的最佳量为1重量%。在阳光照射下,1 wt%Si-CQDs / TiO 2复合材料能够在240分钟内完全降解5 mg / L对乙酰氨基酚(比纯TiO 2快33.3%)。复合材料的优异性能归因于Si-CQDs在TiO 2表面上的协同作用,起到光敏剂和电子陷阱的作用。Si-CQD扩展了TiO 2的光吸收通过将带隙能量从3.20 eV降低到3.12 eV,已通过UV-vis漫反射光谱(DRS)光谱证实。光致发光(PL)光谱和N 2吸附等温线表明,添加Si-CQDs可以延长电荷分离的寿命并改善表面积(比TiO 2大17%)。Si-CQDs / TiO 2的复合材料还显示出良好的稳定性,这有利于将来去除制药废物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号