Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.jphotochem.2020.112425 Leila Nassaji Jahromi , Reza Fazaeli , Reza Behjatmanesh-Ardakani , Mehdi Taghdiri
|
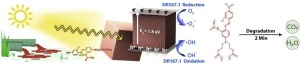
The monoazo dye Disperse Red 167.1 (DR167.1) is known as a carcinogenic pollutant in industrial textile wastewater. In the present study, cubic Cu2O morphology was synthesized and characterized by FESEM, XRD, BET / BJH, FTIR, and DRS techniques. We found the crystallite size was 24.96 nm by the Williamson-Hall. Also, the band gap energy was equal to 1.8 eV by Kubelka-Munk. To further understand the general structure and electron properties, theoretical density calculations were performed using DOML3, CASTEP and FHI- aims codes. The direct band gap was 1.298eV by (CASTEP) code and 1.886 eV by (FHI aims) code, which was calculated using Principle Density Function (DFT) calculations. We found that based on the selection rule (ΔL=±1), two types of electronic transitions are possible: Electron transition from 3dCu to 3pCu orbitals and 2pO to 3dCu orbitals. Physicochemical parameters were calculated in photocatalytic degradation of DR167.1 by Cu2O nanoparticles. The oxidation results indicated that at pH equal to 6.44 without the presence of H2O2, 0.75 mg.L−1 of Cu2O, and color concentration of 210 mg.L−1 during 2 min, a considerable efficiency as large as 84.40 % was obtained. Removal under the visible light irradiation rose to 93.25% upon increasing the time to 40 min. The kinetics of this process obeyed from pseudo-second order model. From an isothermic point of view, the highest correlations and minimum error values belonged to the Fritz Schlunder, Koble Corrigan, and Tempkin isotherms respectively.
中文翻译:

立方Cu 2 O纳米粒子对分散红167.1的光催化矿化作用:实验和理论方法
单偶氮染料Disperse Red 167.1(DR167.1)被称为工业纺织品废水中的致癌污染物。在本研究中,通过FESEM,XRD,BET / BJH,FTIR和DRS技术合成并表征了立方Cu 2 O形态。我们发现威廉姆森-霍尔的微晶尺寸为24.96 nm。此外,Kubelka-Munk的带隙能量等于1.8 eV。为了进一步了解一般结构和电子性质,使用DOML 3进行了理论密度计算,CASTEP和FHI-目标代码。直接带隙通过(CASTEP)代码为1.298eV,通过(FHI目标)代码为1.886 eV,这是使用原理密度函数(DFT)计算得出的。我们发现,基于选择规则(ΔL=±1),两种类型的电子跃迁是可能的:从3dCu到3pCu轨道的电子跃迁以及从2pO到3dCu轨道的电子跃迁。计算了Cu 2 O纳米粒子光催化降解DR167.1的理化参数。氧化反应结果表明,在pH等于6.44无H的存在2 Ò 2,0.75 mg.L -1的铜2 O,和210 mg.L的颜色浓度-1在2分钟内,获得了高达84.40%的显着效率。将时间延长至40分钟后,可见光照射下的去除率升至93.25%。该过程的动力学服从伪二级模型。从等温线的角度来看,最高相关性和最小误差值分别属于Fritz Schlunder,Koble Corrigan和Tempkin等温线。











































 京公网安备 11010802027423号
京公网安备 11010802027423号