Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.comptc.2020.112742 Nicolás D. Gómez , M. Laura Azcárate , Jorge Codnia , Carlos J. Cobos
|
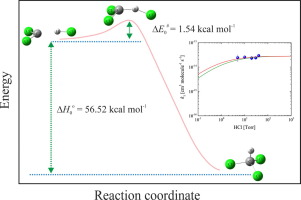
The CCl2 + HCl → CHCl3 (1) insertion reaction has been investigated by ab initio molecular orbital and kinetic calculations. The geometries of reactants, products and transition state were optimized with a large number of density functional theory (DFT) formulations combined with the 6-311++G(3df,3pd) basis set. The reaction barriers calculated with G4(MP2)//DFT and G4//DFT theories of 1.16 ± 0.27 and 0.61 ± 0.26 kcal mol-1 are consistent with the barrier height of ΔE0# = 1.54 ± 0.30 kcal mol-1 estimated from the room temperature experimental rate constant. Calculated enthalpies of reaction of -53.97 ± 0.09 (G4(MP2)//DFT), -55.27 ± 0.09 (G4//DFT) and -56.52 ± 3.90 kcal mol-1 (DFT) agree well with experimental values. The obtained rate constants over the 300 - 2000 K temperature range at the high and low pressure limit can be expressed as k1,∞(G4(MP2)//DFT) = (4.8 ± 1.8) x 10-14 (T/300 K)2.12±0.19 and k1,∞(G4//DFT) = (9.8 ± 3.4) x 10-14 (T/300 K)1.77±0.16 in cm3 molecule-1 s-1, and k1,0 = [HCl] 3.40 x 10-27 (T/300 K)-6.57 exp(-2218 K/T) cm3 molecule-1 s-1. Falloff curves for the intermediate pressure range obtained for the reactions (1, -1) with these rate constants and a derived central broadening factor of Fcent = 0.16 + 0.84 exp(-T/331 K) + exp(-7860 K/T) were compared with reported experimental rate data.
中文翻译:

CCl 2 + HCl→CHCl 3插入反应的量子化学和动力学研究
通过从头算分子轨道和动力学计算,已经研究了CCl 2 + HCl→CHCl 3(1)的插入反应。使用大量的密度泛函理论(DFT)公式结合6-311 ++ G(3 df,3 pd)基础集对反应物,产物和过渡态的几何形状进行了优化。用G4(MP2)// DFT和G4 // DFT理论计算的反应势垒为1.16±0.27和0.61±0.26 kcal mol -1与势垒高度ΔE 0 # = 1.54±0.30 kcal mol -1一致从室温估算实验速率常数。计算的-53.97±0.09(G4(MP2)// DFT),-55.27±0.09(G4 // DFT)和-56.52±3.90kcal mol -1(DFT)的反应焓与实验值很好地吻合。在高压和低压极限下在300-2000 K温度范围内获得的速率常数可以表示为k 1,∞(G4(MP2)// DFT)=(4.8±1.8)x 10 -14(T / 300 K)2.12±0.19和k 1,∞(G4 // DFT)=(9.8±3.4)x 10 -14(T / 300 K)1.77±0.16 in cm 3分子-1 s -1,以及k 1,0 = [HCl] 3.40 x 10 -27(T / 300 K)-6.57 exp(-2218 K / T)cm 3分子-1 s -1。具有这些速率常数和派生的中心展宽因子F cent = 0.16 + 0.84 exp(-T / 331 K)+ exp(-7860 K / T)的反应(1,-1)获得的中压范围的衰减曲线)与报告的实验速率数据进行了比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号