当前位置:
X-MOL 学术
›
Nat. Rev. Urol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Checkpoint inhibitor immunotherapy in kidney cancer.
Nature Reviews Urology ( IF 12.1 ) Pub Date : 2020-02-04 , DOI: 10.1038/s41585-020-0282-3 Wenxin Xu 1 , Michael B Atkins 2 , David F McDermott 1
Nature Reviews Urology ( IF 12.1 ) Pub Date : 2020-02-04 , DOI: 10.1038/s41585-020-0282-3 Wenxin Xu 1 , Michael B Atkins 2 , David F McDermott 1
Affiliation
|
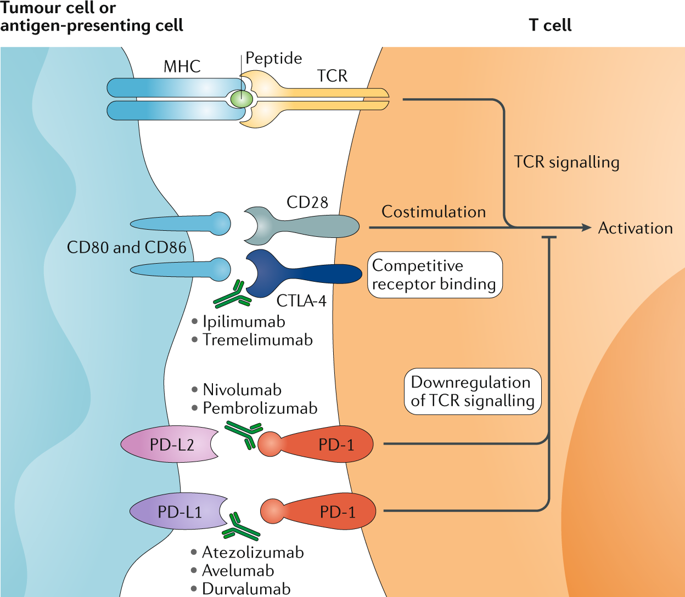
Kidney cancer has unique features that make this malignancy attractive for therapeutic approaches that target components of the immune system. Immune checkpoint inhibition is a well-established part of kidney cancer treatment, and rapid advances continue to be made in this field. Initial preclinical studies that elucidated the biology of the programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) immune checkpoints led to a series of clinical trials that resulted in regulatory approval of nivolumab and the combination of ipilimumab plus nivolumab for the treatment of advanced renal cell carcinoma. Subsequent data led to approvals of combination strategies of immune checkpoint inhibition plus agents that target the vascular endothelial growth factor receptor and a shift in the current standard of renal cell carcinoma care. However, controversies remain regarding the optimal therapy selection and treatment strategy for individual patients, which might be eventually overcome by current intensive efforts in biomarker research. That work includes evaluation of tumour cell PD-L1 expression, gene expression signatures, CD8+ T cell density and others. In the future, further advances in the understanding of immune checkpoint biology might reveal new therapeutic targets beyond PD-1, PD-L1 and CTLA-4, as well as new combination approaches.
中文翻译:

肾癌的检查点抑制剂免疫疗法。
肾脏癌具有独特的功能,使这种恶性肿瘤成为靶向免疫系统成分的治疗方法的诱人之处。免疫检查点抑制是肾癌治疗中公认的一部分,并且在该领域中继续快速发展。初步临床前研究阐明了程序性细胞死亡1(PD-1),程序性细胞死亡1配体1(PD-L1)和细胞毒性T淋巴细胞抗原4(CTLA-4)免疫检查点的生物学性,从而进行了一系列临床试验获得了nivolumab的监管批准,以及ipilimumab和nivolumab的组合治疗晚期肾细胞癌。随后的数据导致批准了免疫检查点抑制与靶向血管内皮生长因子受体的药物联合治疗的组合策略,并改变了当前肾细胞癌治疗标准。然而,针对个体患者的最佳治疗选择和治疗策略仍存在争议,目前在生物标志物研究中的大量努力最终可能会克服这些争议。这项工作包括评估肿瘤细胞PD-L1的表达,基因表达特征,CD8 + T细胞密度等。将来,对免疫检查点生物学的进一步了解可能会揭示出PD-1,PD-L1和CTLA-4以外的新治疗靶标,以及新的联合治疗方法。关于针对单个患者的最佳治疗选择和治疗策略的争议仍然存在,目前在生物标记物研究中的大量努力最终可能会克服这些争议。这项工作包括评估肿瘤细胞PD-L1的表达,基因表达特征,CD8 + T细胞密度等。将来,对免疫检查点生物学的进一步了解可能会揭示出PD-1,PD-L1和CTLA-4以外的新治疗靶标,以及新的联合治疗方法。关于针对单个患者的最佳治疗选择和治疗策略的争议仍然存在,目前在生物标记物研究中的大量努力最终可能会克服这些争议。这项工作包括评估肿瘤细胞PD-L1的表达,基因表达特征,CD8 + T细胞密度等。将来,对免疫检查点生物学的进一步了解可能会揭示出PD-1,PD-L1和CTLA-4以外的新治疗靶标,以及新的联合治疗方法。
更新日期:2020-02-04
中文翻译:

肾癌的检查点抑制剂免疫疗法。
肾脏癌具有独特的功能,使这种恶性肿瘤成为靶向免疫系统成分的治疗方法的诱人之处。免疫检查点抑制是肾癌治疗中公认的一部分,并且在该领域中继续快速发展。初步临床前研究阐明了程序性细胞死亡1(PD-1),程序性细胞死亡1配体1(PD-L1)和细胞毒性T淋巴细胞抗原4(CTLA-4)免疫检查点的生物学性,从而进行了一系列临床试验获得了nivolumab的监管批准,以及ipilimumab和nivolumab的组合治疗晚期肾细胞癌。随后的数据导致批准了免疫检查点抑制与靶向血管内皮生长因子受体的药物联合治疗的组合策略,并改变了当前肾细胞癌治疗标准。然而,针对个体患者的最佳治疗选择和治疗策略仍存在争议,目前在生物标志物研究中的大量努力最终可能会克服这些争议。这项工作包括评估肿瘤细胞PD-L1的表达,基因表达特征,CD8 + T细胞密度等。将来,对免疫检查点生物学的进一步了解可能会揭示出PD-1,PD-L1和CTLA-4以外的新治疗靶标,以及新的联合治疗方法。关于针对单个患者的最佳治疗选择和治疗策略的争议仍然存在,目前在生物标记物研究中的大量努力最终可能会克服这些争议。这项工作包括评估肿瘤细胞PD-L1的表达,基因表达特征,CD8 + T细胞密度等。将来,对免疫检查点生物学的进一步了解可能会揭示出PD-1,PD-L1和CTLA-4以外的新治疗靶标,以及新的联合治疗方法。关于针对单个患者的最佳治疗选择和治疗策略的争议仍然存在,目前在生物标记物研究中的大量努力最终可能会克服这些争议。这项工作包括评估肿瘤细胞PD-L1的表达,基因表达特征,CD8 + T细胞密度等。将来,对免疫检查点生物学的进一步了解可能会揭示出PD-1,PD-L1和CTLA-4以外的新治疗靶标,以及新的联合治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号