当前位置:
X-MOL 学术
›
Neurobiol. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
UsnRNP trafficking is regulated by stress granules and compromised by mutant ALS proteins.
Neurobiology of Disease ( IF 5.1 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.nbd.2020.104792 Simona Rossi 1 , Valentina Rompietti 2 , Ylenia Antonucci 2 , Daniela Giovannini 2 , Chiara Scopa 3 , Silvia Scaricamazza 4 , Raffaella Scardigli 5 , Gianluca Cestra 6 , Annalucia Serafino 2 , Maria Teresa Carrì 4 , Nadia D'Ambrosi 4 , Mauro Cozzolino 2
Neurobiology of Disease ( IF 5.1 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.nbd.2020.104792 Simona Rossi 1 , Valentina Rompietti 2 , Ylenia Antonucci 2 , Daniela Giovannini 2 , Chiara Scopa 3 , Silvia Scaricamazza 4 , Raffaella Scardigli 5 , Gianluca Cestra 6 , Annalucia Serafino 2 , Maria Teresa Carrì 4 , Nadia D'Ambrosi 4 , Mauro Cozzolino 2
Affiliation
|
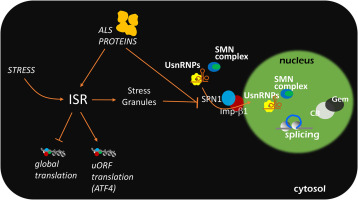
Activation of the integrated stress response (ISR), alterations in nucleo-cytoplasmic (N/C) transport and changes in alternative splicing regulation are all common traits of the pathogenesis of Amyotrophic Lateral Sclerosis (ALS). However, whether these processes act independently from each other, or are part of a coordinated mechanism of gene expression regulation that is affected in pathogenic conditions, is still rather undefined. To answer these questions, in this work we set out to characterise the functional connections existing between ISR activation and nucleo-cytosol trafficking and nuclear localization of spliceosomal U-rich small nuclear ribonucleoproteins (UsnRNPs), the core constituents of the spliceosome, and to study how ALS-linked mutant proteins affect this interplay. Activation of the ISR induces a profound reorganization of nuclear Gems and Cajal bodies, the membrane-less particles that assist UsnRNP maturation and storage. This effect requires the cytoplasmic assembly of SGs and is associated to the disturbance of the nuclear import of UsnRNPs by the snurportin-1/importin-β1 system. Notably, these effects are reversed by both inhibiting the ISR or upregulating importin-β1. This indicates that SGs are major determinants of Cajal bodies assembly and that the modulation of N/C trafficking of UsnRNPs might control alternative splicing in response to stress. Importantly, the dismantling of nuclear Gems and Cajal bodies by ALS-linked mutant FUS or C9orf72-derived dipeptide repeat proteins is halted by overexpression of importin-β1, but not by inhibition of the ISR. This suggests that changes in the nuclear localization of the UsnRNP complexes induced by mutant ALS proteins are uncoupled from ISR activation, and that defects in the N/C trafficking of UsnRNPs might play a role in ALS pathogenesis.
中文翻译:

UsnRNP的运输受到应激颗粒的调节,并受到突变ALS蛋白的破坏。
整合应激反应(ISR)的激活,核质(N / C)转运的改变以及可变剪接调控的改变都是肌萎缩性侧索硬化症(ALS)发病机制的常见特征。然而,这些过程是否彼此独立起作用,还是在致病条件下影响基因表达调控的协调机制的一部分,仍然是不确定的。为了回答这些问题,在这项工作中,我们着手表征ISR活化与核质溶胶运输和剪接体富含U的小核糖核蛋白(UsnRNPs)(剪接体的核心组成部分)之间的功能连接之间存在的功能,并进行研究ALS连锁突变蛋白如何影响这种相互作用。ISR的激活引起核宝石和Cajal体的深刻重组,这是无膜的颗粒,有助于UsnRNP的成熟和储存。该作用需要SGs的细胞质组装,并与通过snurportin-1 /importin-β1系统干扰UsnRNPs的核输入有关。明显地,这些作用通过抑制ISR或上调importin-β1而被逆转。这表明SGs是Cajal体组装的主要决定因素,而UsnRNPs的N / C转运的调节可能控制对应激的替代剪接。重要的是,ALS连接的突变体FUS或C9orf72衍生的二肽重复蛋白对核宝石和Cajal体的分解可通过importin-β1的过表达来停止,但不能通过抑制ISR来停止。
更新日期:2020-02-04
中文翻译:

UsnRNP的运输受到应激颗粒的调节,并受到突变ALS蛋白的破坏。
整合应激反应(ISR)的激活,核质(N / C)转运的改变以及可变剪接调控的改变都是肌萎缩性侧索硬化症(ALS)发病机制的常见特征。然而,这些过程是否彼此独立起作用,还是在致病条件下影响基因表达调控的协调机制的一部分,仍然是不确定的。为了回答这些问题,在这项工作中,我们着手表征ISR活化与核质溶胶运输和剪接体富含U的小核糖核蛋白(UsnRNPs)(剪接体的核心组成部分)之间的功能连接之间存在的功能,并进行研究ALS连锁突变蛋白如何影响这种相互作用。ISR的激活引起核宝石和Cajal体的深刻重组,这是无膜的颗粒,有助于UsnRNP的成熟和储存。该作用需要SGs的细胞质组装,并与通过snurportin-1 /importin-β1系统干扰UsnRNPs的核输入有关。明显地,这些作用通过抑制ISR或上调importin-β1而被逆转。这表明SGs是Cajal体组装的主要决定因素,而UsnRNPs的N / C转运的调节可能控制对应激的替代剪接。重要的是,ALS连接的突变体FUS或C9orf72衍生的二肽重复蛋白对核宝石和Cajal体的分解可通过importin-β1的过表达来停止,但不能通过抑制ISR来停止。











































 京公网安备 11010802027423号
京公网安备 11010802027423号