当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Disulfiram causes selective hypoxic cancer cell toxicity and radio-chemo-sensitization via redox cycling of copper.
Free Radical Biology and Medicine ( IF 7.4 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.freeradbiomed.2020.01.186 Kelly C Falls-Hubert 1 , Aimee L Butler 1 , Kai Gui 1 , Michael Anderson 1 , Mengshi Li 2 , Jeffrey M Stolwijk 1 , Samuel N Rodman 1 , Shane R Solst 1 , Ann Tomanek-Chalkley 1 , Charles C Searby 3 , Val C Sheffield 3 , Vanessa Sandfort 4 , Hartmut Schmidt 4 , Michael L McCormick 1 , Brian R Wels 5 , Bryan G Allen 1 , Garry R Buettner 1 , Michael K Schultz 6 , Douglas R Spitz 1
Free Radical Biology and Medicine ( IF 7.4 ) Pub Date : 2020-02-04 , DOI: 10.1016/j.freeradbiomed.2020.01.186 Kelly C Falls-Hubert 1 , Aimee L Butler 1 , Kai Gui 1 , Michael Anderson 1 , Mengshi Li 2 , Jeffrey M Stolwijk 1 , Samuel N Rodman 1 , Shane R Solst 1 , Ann Tomanek-Chalkley 1 , Charles C Searby 3 , Val C Sheffield 3 , Vanessa Sandfort 4 , Hartmut Schmidt 4 , Michael L McCormick 1 , Brian R Wels 5 , Bryan G Allen 1 , Garry R Buettner 1 , Michael K Schultz 6 , Douglas R Spitz 1
Affiliation
|
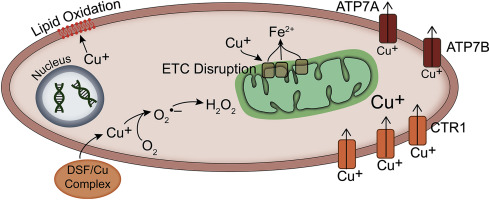
Therapies for lung cancer patients initially elicit desirable responses, but the presence of hypoxia and drug resistant cells within tumors ultimately lead to treatment failure. Disulfiram (DSF) is an FDA approved, copper chelating agent that can target oxidative metabolic frailties in cancer vs. normal cells and be repurposed as an adjuvant to cancer therapy. Clonogenic survival assays showed that DSF (50-150 nM) combined with physiological levels of Cu (15 μM CuSO4) was selectively toxic to H292 NSCLC cells vs. normal human bronchial epithelial cells (HBEC). Furthermore, cancer cell toxicity was exacerbated at 1% O2, relative to 4 or 21% O2. This selective toxicity of DSF/Cu was associated with differential Cu ionophore capabilities. DSF/Cu treatment caused a >20-fold increase in cellular Cu in NSCLCs, with nearly two-fold higher Cu present in NSCLCs vs. HBECs and in cancer cells at 1% O2vs. 21% O2. DSF toxicity was shown to be dependent on the retention of Cu as well as oxidative stress mechanisms, including the production of superoxide, peroxide, lipid peroxidation, and mitochondrial damage. DSF was also shown to selectively (relative to HBECs) enhance radiation and chemotherapy-induced NSCLC killing and reduce radiation and chemotherapy resistance in hypoxia. Finally, DSF decreased xenograft tumor growth in vivo when combined with radiation and carboplatin. These results support the hypothesis that DSF could be a promising adjuvant to enhance cancer therapy based on its apparent ability to selectively target fundamental differences in cancer cell oxidative metabolism.
中文翻译:

双硫仑通过铜的氧化还原循环导致选择性缺氧癌细胞毒性和放射化学增敏作用。
肺癌患者的疗法最初会引起理想的反应,但肿瘤内缺氧和耐药细胞的存在最终导致治疗失败。双硫仑(DSF)是FDA批准的铜螯合剂,可靶向癌症相对于正常细胞的氧化代谢弱点,并重新用作癌症治疗的佐剂。克隆存活试验表明,与正常人支气管上皮细胞(HBEC)相比,DSF(50-150 nM)结合生理水平的Cu(15μMCuSO4)对H292 NSCLC细胞有选择性毒性。此外,相对于4%或21%的O2,1%的O2会加剧癌细胞的毒性。DSF / Cu的选择性毒性与Cu离子载体的不同能力有关。DSF / Cu处理导致NSCLC中细胞Cu的增加> 20倍,在非小细胞肺癌和癌细胞中,铜的含量是1%O2vs的两倍。氧气含量为21%。已证明DSF毒性取决于Cu的保留以及氧化应激机制,包括超氧化物,过氧化物,脂质过氧化和线粒体损伤的产生。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。已证明DSF毒性取决于Cu的保留以及氧化应激机制,包括超氧化物,过氧化物,脂质过氧化和线粒体损伤的产生。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。已证明DSF毒性取决于Cu的保留以及氧化应激机制,包括超氧化物,过氧化物,脂质过氧化和线粒体损伤的产生。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。和线粒体损害。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。和线粒体损害。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。
更新日期:2020-02-04
中文翻译:

双硫仑通过铜的氧化还原循环导致选择性缺氧癌细胞毒性和放射化学增敏作用。
肺癌患者的疗法最初会引起理想的反应,但肿瘤内缺氧和耐药细胞的存在最终导致治疗失败。双硫仑(DSF)是FDA批准的铜螯合剂,可靶向癌症相对于正常细胞的氧化代谢弱点,并重新用作癌症治疗的佐剂。克隆存活试验表明,与正常人支气管上皮细胞(HBEC)相比,DSF(50-150 nM)结合生理水平的Cu(15μMCuSO4)对H292 NSCLC细胞有选择性毒性。此外,相对于4%或21%的O2,1%的O2会加剧癌细胞的毒性。DSF / Cu的选择性毒性与Cu离子载体的不同能力有关。DSF / Cu处理导致NSCLC中细胞Cu的增加> 20倍,在非小细胞肺癌和癌细胞中,铜的含量是1%O2vs的两倍。氧气含量为21%。已证明DSF毒性取决于Cu的保留以及氧化应激机制,包括超氧化物,过氧化物,脂质过氧化和线粒体损伤的产生。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。已证明DSF毒性取决于Cu的保留以及氧化应激机制,包括超氧化物,过氧化物,脂质过氧化和线粒体损伤的产生。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。已证明DSF毒性取决于Cu的保留以及氧化应激机制,包括超氧化物,过氧化物,脂质过氧化和线粒体损伤的产生。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。和线粒体损害。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。和线粒体损害。还显示出DSF选择性(相对于HBECs)增强放射线和化疗引起的NSCLC杀伤力,并降低缺氧时的放射线和化疗耐药性。最后,当与放射线和卡铂联合使用时,DSF降低了体内异种移植肿瘤的生长。这些结果支持以下假设:DSF可以选择性地靶向癌细胞氧化代谢中的基本差异,具有明显的能力,可以作为增强癌症治疗的有希望的佐剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号