Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.jcat.2020.01.003 Pengyu Xu , Shilpa Agarwal , Leon Lefferts
|
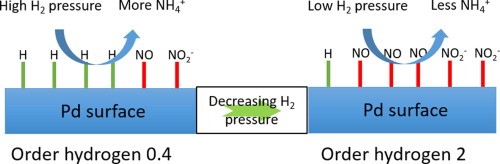
The kinetics of nitrite hydrogenation over a Pd/γ-Al2O3 catalyst was studied in a semi-batch slurry reactor at atmospheric pressure, in absence of any mass transfer effects. The hydrogen concentration and pH were kept constant during an experiment by continuously flowing a gas mixture containing hydrogen and 10% v/v CO2. The kinetic experiments were performed in an unprecedented wide concentration window of nitrite and hydrogen, revealing extreme variation in the apparent orders in hydrogen and nitrite, including reaction orders in hydrogen between 2 and 0.3, whereas the order in nitrite varied between 0.4 and −0.9. The rate of reaction is almost exclusively determined by the rate of formation of N2 as the selectivity to ammonia is very low. A Langmuir-Hinshelwood mechanism with competitive adsorption is in operation. Several mechanistic pathways, as well as possible rate determining steps in those pathways, are discussed based on these observations in combination with prior knowledge on the mechanism in literature, resulting in a revised mechanistic scheme. It is concluded that formation of NH via dissociative hydrogenation of HNOH is the rate determining step, whereas molecular N2 forms via reaction of NH with either NO, NOH or HNOH. N-N bond formation via dimerization of adsorbed NO or adsorbed N can be excluded.
中文翻译:

亚硝酸盐氢化在Pd /机理的γ-Al 2 ö 3根据一个严格的动力学研究
亚硝酸盐氢化的在钯/γ-Al系的动力学2 ö 3催化剂在大气压下半分批淤浆反应器进行了研究,在不存在任何质量转移的效果。在实验期间,通过使含有氢和10%v / v CO 2的气体混合物连续流动,使氢浓度和pH保持恒定。在亚硝酸盐和氢的前所未有的宽浓度窗口中进行了动力学实验,揭示了氢和亚硝酸盐的表观次序的极端变化,包括氢在2到0.3之间的反应次序,而亚硝酸盐在0.4到-0.9之间的次序变化。反应速率几乎完全由N 2的形成速率决定因为对氨的选择性很低。具有竞争吸附作用的Langmuir-Hinshelwood机理正在运行。基于这些观察结果,结合文献中对该机制的先验知识,讨论了几种机制途径以及这些途径中可能的速率确定步骤,从而提出了一种改进的机制方案。结论是,通过HNOH的解离氢化形成NH是速率决定步骤,而分子N 2是通过NH与NO,NOH或HNOH反应形成的。可以排除通过吸附的NO或吸附的N的二聚作用形成的NN键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号