当前位置:
X-MOL 学术
›
Eur. J. Pharm. Biopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diffusion Modelling of Percutaneous Absorption Kinetics. Predicting urinary excretion from in vitro skin permeation tests (IVPT) for an infinite dose.
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.ejpb.2020.01.018 Xin Liu 1 , Shereen Yousef 2 , Yuri G Anissimov 3 , John van der Hoek 4 , Eleftheria Tsakalozou 5 , Zhanglin Ni 5 , Jeffrey E Grice 1 , Michael S Roberts 6
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.ejpb.2020.01.018 Xin Liu 1 , Shereen Yousef 2 , Yuri G Anissimov 3 , John van der Hoek 4 , Eleftheria Tsakalozou 5 , Zhanglin Ni 5 , Jeffrey E Grice 1 , Michael S Roberts 6
Affiliation
|
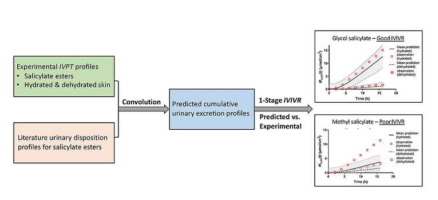
In this work, we developed a number of generalised skin diffusion based pharmacokinetic models to relate published in vivo urinary excretion data to matching experimentally generated in vitro human skin permeation test (IVPT) data for a series of topically applied salicylate esters. A simplified linear in vivo model was found to inadequately describe the time course of urinary excretion over the entire sampling period. We represented the skin barrier as both a one layer (stratum corneum) and a two-layer (stratum corneum with viable epidermis) diffusion model and convoluted their Laplace solutions with that for a single exponential disposition phase to describe the urinary excretion profiles in the Laplace domain. We also derived asymptotic approximations for the model and estimated the conditions under which they could be used. We then sought to develop in vitro - in vivo relationships (IVIVR) for topically applied methyl, ethyl and glycol salicylates using our experimental IVPT data and the literature urinary excretion data. Good linear IVIVRs for ethyl and glycol salicylates were obtained, but the IVIVR for methyl salicylate was poor, perhaps because of topical stimulation of local skin blood flow by methyl salicylate. The ratio of the hydrated to dehydrated skin permeation for all salicylate esters was the same in both the IVPT and in vivo studies. A diffusion based one compartment pharmacokinetic model was also developed to describe the urinary excretion of solutes after removal of topical products and to compare the methyl salicylate skin permeation for five different body sites. The work presented here is consistent with the development of skin IVIVRs, but suggest that different skin conditions, application sites and local skin effects may affect model predictions.
中文翻译:

经皮吸收动力学的扩散模型。通过无限量的体外皮肤渗透测试(IVPT)预测尿液排泄。
在这项工作中,我们开发了许多基于皮肤扩散的广义药代动力学模型,以将已发表的体内尿液排泄数据与一系列局部应用的水杨酸酯的实验生成的体外人体皮肤渗透测试(IVPT)数据进行匹配。发现简化的线性体内模型不足以描述整个采样期间尿液排泄的时间过程。我们将皮肤屏障表示为一层(角质层)和两层(具有存活表皮的角质层)扩散模型,并将其Laplace解决方案与单个指数处置阶段的溶液进行卷积,以描述Laplace中的尿液排泄曲线域。我们还导出了该模型的渐近近似值,并估计了可以使用它们的条件。然后,我们尝试使用我们的实验IVPT数据和文献尿液排泄数据来开发局部应用的甲基,乙基和乙二醇水杨酸酯的体外-体内关系(IVIVR)。水杨酸乙酯和水杨酸乙二醇酯的线性IVIVR良好,但水杨酸甲酯的IVIVR较差,这可能是因为水杨酸甲酯可局部刺激局部皮肤血流。在IVPT和体内研究中,所有水杨酸酯的水合和脱水皮肤渗透率均相同。还开发了一种基于扩散的一室药代动力学模型,以描述去除局部产品后尿液中溶质的排泄,并比较五个不同身体部位的水杨酸甲酯皮肤渗透率。此处介绍的工作与皮肤IVIVR的开发一致,
更新日期:2020-02-03
中文翻译:

经皮吸收动力学的扩散模型。通过无限量的体外皮肤渗透测试(IVPT)预测尿液排泄。
在这项工作中,我们开发了许多基于皮肤扩散的广义药代动力学模型,以将已发表的体内尿液排泄数据与一系列局部应用的水杨酸酯的实验生成的体外人体皮肤渗透测试(IVPT)数据进行匹配。发现简化的线性体内模型不足以描述整个采样期间尿液排泄的时间过程。我们将皮肤屏障表示为一层(角质层)和两层(具有存活表皮的角质层)扩散模型,并将其Laplace解决方案与单个指数处置阶段的溶液进行卷积,以描述Laplace中的尿液排泄曲线域。我们还导出了该模型的渐近近似值,并估计了可以使用它们的条件。然后,我们尝试使用我们的实验IVPT数据和文献尿液排泄数据来开发局部应用的甲基,乙基和乙二醇水杨酸酯的体外-体内关系(IVIVR)。水杨酸乙酯和水杨酸乙二醇酯的线性IVIVR良好,但水杨酸甲酯的IVIVR较差,这可能是因为水杨酸甲酯可局部刺激局部皮肤血流。在IVPT和体内研究中,所有水杨酸酯的水合和脱水皮肤渗透率均相同。还开发了一种基于扩散的一室药代动力学模型,以描述去除局部产品后尿液中溶质的排泄,并比较五个不同身体部位的水杨酸甲酯皮肤渗透率。此处介绍的工作与皮肤IVIVR的开发一致,











































 京公网安备 11010802027423号
京公网安备 11010802027423号