当前位置:
X-MOL 学术
›
Transl. Psychiaty
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rivastigmine modifies the α-secretase pathway and potentially early Alzheimer's disease.
Translational Psychiatry ( IF 5.8 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41398-020-0709-x Balmiki Ray 1 , Bryan Maloney 1, 2 , Kumar Sambamurti 3 , Hanuma K Karnati 4 , Peter T Nelson 5 , Nigel H Greig 4 , Debomoy K Lahiri 1, 2, 6
Translational Psychiatry ( IF 5.8 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41398-020-0709-x Balmiki Ray 1 , Bryan Maloney 1, 2 , Kumar Sambamurti 3 , Hanuma K Karnati 4 , Peter T Nelson 5 , Nigel H Greig 4 , Debomoy K Lahiri 1, 2, 6
Affiliation
|
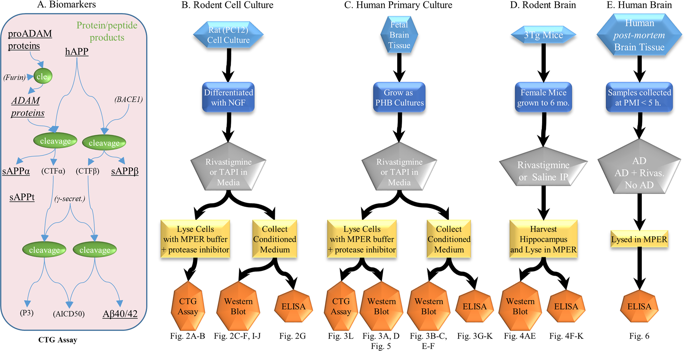
Rivastigmine (or Exelon) is a cholinesterase inhibitor, currently used as a symptomatic treatment for mild-to-moderate Alzheimer's disease (AD). Amyloid-β peptide (Aβ) generated from its precursor protein (APP) by β-secretase (or BACE1) and γ-secretase endoproteolysis. Alternative APP cleavage by α-secretase (a family of membrane-bound metalloproteases- Adamalysins) precludes the generation of toxic Aβ and yields a neuroprotective and neurotrophic secreted sAPPα fragment. Several signal transduction pathways, including protein kinase C and MAP kinase, stimulate α-secretase. We present data to suggest that rivastigmine, in addition to anticholinesterase activity, directs APP processing away from BACE1 and towards α-secretases. We treated rat neuronal PC12 cells and primary human brain (PHB) cultures with rivastigmine and the α-secretase inhibitor TAPI and assayed for levels of APP processing products and α-secretases. We subsequently treated 3×Tg (transgenic) mice with rivastigmine and harvested hippocampi to assay for levels of APP processing products. We also assayed postmortem human control, AD, and AD brains from subjects treated with rivastigmine for levels of APP metabolites. Rivastigmine dose-dependently promoted α-secretase activity by upregulating levels of ADAM-9, -10, and -17 α-secretases in PHB cultures. Co-treatment with TAPI eliminated rivastigmine-induced sAPPα elevation. Rivastigmine treatment elevated levels of sAPPα in 3×Tg mice. Consistent with these results, we also found elevated sAPPα in postmortem brain samples from AD patients treated with rivastigmine. Rivastigmine can modify the levels of several shedding proteins and directs APP processing toward the non-amyloidogenic pathway. This novel property of rivastigmine can be therapeutically exploited for disease-modifying intervention that goes beyond symptomatic treatment for AD.
中文翻译:

卡巴拉汀改变 α-分泌酶途径和潜在的早期阿尔茨海默病。
卡巴拉汀(或 Exelon)是一种胆碱酯酶抑制剂,目前用作轻度至中度阿尔茨海默病(AD)的对症治疗。β-分泌酶 (或 BACE1) 和 γ-分泌酶内切蛋白酶从其前体蛋白 (APP) 生成的淀粉样蛋白-β 肽 (Aβ)。由 α-分泌酶(膜结合金属蛋白酶 - Adamalysins 家族)进行的替代 APP 切割排除了有毒 Aβ 的产生,并产生了神经保护和神经营养的分泌 sAPPα 片段。几种信号转导途径,包括蛋白激酶 C 和 MAP 激酶,可刺激 α-分泌酶。我们提供的数据表明,除抗胆碱酯酶活性外,卡巴拉汀还将 APP 加工从 BACE1 引导到 α-分泌酶。我们用卡巴拉汀和 α-分泌酶抑制剂 TAPI 处理大鼠神经元 PC12 细胞和原代人脑 (PHB) 培养物,并测定 APP 加工产物和 α-分泌酶的水平。我们随后用卡巴拉汀治疗 3×Tg(转基因)小鼠并收获海马体以测定 APP 加工产物的水平。我们还检测了接受卡巴拉汀治疗的受试者的死后人类对照、AD 和 AD 大脑的 APP 代谢物水平。Rivastigmine 通过上调 PHB 培养物中 ADAM-9、-10 和 -17 α-分泌酶的水平,以剂量依赖性方式促进 α-分泌酶活性。与 TAPI 共同治疗消除了卡巴拉汀诱导的 sAPPα 升高。卡巴拉汀治疗提高了 3×Tg 小鼠的 sAPPα 水平。与这些结果一致,我们还在接受卡巴拉汀治疗的 AD 患者的死后脑样本中发现了升高的 sAPPα。卡巴拉汀可以改变几种脱落蛋白的水平,并将 APP 加工导向非淀粉样蛋白生成途径。卡巴拉汀的这种新特性可以在治疗上用于改善疾病的干预,超出对 AD 的对症治疗。
更新日期:2020-02-03
中文翻译:

卡巴拉汀改变 α-分泌酶途径和潜在的早期阿尔茨海默病。
卡巴拉汀(或 Exelon)是一种胆碱酯酶抑制剂,目前用作轻度至中度阿尔茨海默病(AD)的对症治疗。β-分泌酶 (或 BACE1) 和 γ-分泌酶内切蛋白酶从其前体蛋白 (APP) 生成的淀粉样蛋白-β 肽 (Aβ)。由 α-分泌酶(膜结合金属蛋白酶 - Adamalysins 家族)进行的替代 APP 切割排除了有毒 Aβ 的产生,并产生了神经保护和神经营养的分泌 sAPPα 片段。几种信号转导途径,包括蛋白激酶 C 和 MAP 激酶,可刺激 α-分泌酶。我们提供的数据表明,除抗胆碱酯酶活性外,卡巴拉汀还将 APP 加工从 BACE1 引导到 α-分泌酶。我们用卡巴拉汀和 α-分泌酶抑制剂 TAPI 处理大鼠神经元 PC12 细胞和原代人脑 (PHB) 培养物,并测定 APP 加工产物和 α-分泌酶的水平。我们随后用卡巴拉汀治疗 3×Tg(转基因)小鼠并收获海马体以测定 APP 加工产物的水平。我们还检测了接受卡巴拉汀治疗的受试者的死后人类对照、AD 和 AD 大脑的 APP 代谢物水平。Rivastigmine 通过上调 PHB 培养物中 ADAM-9、-10 和 -17 α-分泌酶的水平,以剂量依赖性方式促进 α-分泌酶活性。与 TAPI 共同治疗消除了卡巴拉汀诱导的 sAPPα 升高。卡巴拉汀治疗提高了 3×Tg 小鼠的 sAPPα 水平。与这些结果一致,我们还在接受卡巴拉汀治疗的 AD 患者的死后脑样本中发现了升高的 sAPPα。卡巴拉汀可以改变几种脱落蛋白的水平,并将 APP 加工导向非淀粉样蛋白生成途径。卡巴拉汀的这种新特性可以在治疗上用于改善疾病的干预,超出对 AD 的对症治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号