Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of a clinical decision rule to guide antibiotic prescription in children with suspected lower respiratory tract infection in The Netherlands: A stepped-wedge cluster randomised trial.
PLOS Medicine ( IF 10.5 ) Pub Date : 2020-01-31 , DOI: 10.1371/journal.pmed.1003034 Josephine S van de Maat 1 , Daphne Peeters 2 , Daan Nieboer 3 , Anne-Marie van Wermeskerken 4 , Frank J Smit 5 , Jeroen G Noordzij 6 , Gerdien Tramper-Stranders 7 , Gertjan J A Driessen 2 , Charlie C Obihara 8 , Jeanine Punt 9 , Johan van der Lei 10 , Suzanne Polinder 3 , Henriette A Moll 1 , Rianne Oostenbrink 1
PLOS Medicine ( IF 10.5 ) Pub Date : 2020-01-31 , DOI: 10.1371/journal.pmed.1003034 Josephine S van de Maat 1 , Daphne Peeters 2 , Daan Nieboer 3 , Anne-Marie van Wermeskerken 4 , Frank J Smit 5 , Jeroen G Noordzij 6 , Gerdien Tramper-Stranders 7 , Gertjan J A Driessen 2 , Charlie C Obihara 8 , Jeanine Punt 9 , Johan van der Lei 10 , Suzanne Polinder 3 , Henriette A Moll 1 , Rianne Oostenbrink 1
Affiliation
|
BACKGROUND
Optimising the use of antibiotics is a key component of antibiotic stewardship. Respiratory tract infections (RTIs) are the most common reason for antibiotic prescription in children, even though most of these infections in children under 5 years are viral. This study aims to safely reduce antibiotic prescriptions in children under 5 years with suspected lower RTI at the emergency department (ED), by implementing a clinical decision rule.
METHODS AND FINDINGS
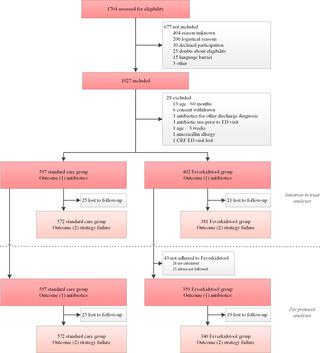
In a stepped-wedge cluster randomised trial, we included children aged 1-60 months presenting with fever and cough or dyspnoea to 8 EDs in The Netherlands. The EDs were of varying sizes, from diverse geographic and demographic regions, and of different hospital types (tertiary versus general). In the pre-intervention phase, children received usual care, according to the Dutch and NICE guidelines for febrile children. During the intervention phase, a validated clinical prediction model (Feverkidstool) including clinical characteristics and C-reactive protein (CRP) was implemented as a decision rule guiding antibiotic prescription. The intervention was that antibiotics were withheld in children with a low or intermediate predicted risk of bacterial pneumonia (≤10%, based on Feverkidstool). Co-primary outcomes were antibiotic prescription rate and strategy failure. Strategy failure was defined as secondary antibiotic prescriptions or hospitalisations, persistence of fever or oxygen dependency up to day 7, or complications. Hospitals were randomly allocated to 1 sequence of treatment each, using computer randomisation. The trial could not be blinded. We used multilevel logistic regression to estimate the effect of the intervention, clustered by hospital and adjusted for time period, age, sex, season, ill appearance, and fever duration; predicted risk was included in exploratory analysis. We included 999 children (61% male, median age 17 months [IQR 9 to 30]) between 1 January 2016 and 30 September 2018: 597 during the pre-intervention phase and 402 during the intervention phase. Most children (77%) were referred by a general practitioner, and half of children were hospitalised. Intention-to-treat analyses showed that overall antibiotic prescription was not reduced (30% to 25%, adjusted odds ratio [aOR] 1.07 [95% CI 0.57 to 2.01, p = 0.75]); strategy failure reduced from 23% to 16% (aOR 0.53 [95% CI 0.32 to 0.88, p = 0.01]). Exploratory analyses showed that the intervention influenced risk groups differently (p < 0.01), resulting in a reduction in antibiotic prescriptions in low/intermediate-risk children (17% to 6%; aOR 0.31 [95% CI 0.12 to 0.81, p = 0.02]) and a non-significant increase in the high-risk group (47% to 59%; aOR 2.28 [95% CI 0.84 to 6.17, p = 0.09]). Two complications occurred during the trial: 1 admission to the intensive care unit during follow-up and 1 pleural empyema at day 10 (both unrelated to the study intervention). Main limitations of the study were missing CRP values in the pre-intervention phase and a prolonged baseline period due to logistical issues, potentially affecting the power of our study.
CONCLUSIONS
In this multicentre ED study, we observed that a clinical decision rule for childhood pneumonia did not reduce overall antibiotic prescription, but that it was non-inferior to usual care. Exploratory analyses showed fewer strategy failures and that fewer antibiotics were prescribed in low/intermediate-risk children, suggesting improved targeting of antibiotics by the decision rule.
TRIAL REGISTRATION
Netherlands Trial Register NTR5326.
中文翻译:

在荷兰,对怀疑下呼吸道感染的儿童进行抗生素处方指导的临床决策规则的评估:楔入式整群随机试验。
背景技术优化抗生素的使用是抗生素管理的关键组成部分。呼吸道感染(RTIs)是儿童服用抗生素处方的最常见原因,尽管5岁以下儿童中的大多数感染是病毒性感染。这项研究旨在通过实施临床决策规则,安全减少急诊室(ED)怀疑RTI较低的5岁以下儿童的抗生素处方。方法和研究结果在荷兰的8例ED中,我们纳入了1到60个月大的有发热,咳嗽或呼吸困难的1至60个月的儿童。急诊室规模大小不一,来自不同的地理和人口区域,并且属于不同的医院类型(三级医院与普通医院)。在干预前阶段,儿童得到了常规照料,根据针对发热儿童的荷兰语和NICE指南。在干预阶段,已实施包括临床特征和C反应蛋白(CRP)在内的经过验证的临床预测模型(Feverkidstool),作为指导抗生素处方的决策规则。干预措施是对细菌性肺炎的低或中等预测风险(≤10%,基于Feverkidstool的儿童)禁用抗生素。共同主要结果是抗生素处方率和策略失败。策略失败定义为继发抗生素处方或住院,直到第7天持续发热或依赖氧气或出现并发症。使用计算机随机化方法将医院随机分配给1种治疗顺序。审判不能盲目。我们使用多级Logistic回归来评估干预措施的效果,并按医院分组并针对时间段,年龄,性别,季节,不适,发烧持续时间进行了调整。预测风险包括在探索性分析中。我们纳入了2016年1月1日至2018年9月30日期间的999名儿童(男61%,中位年龄17个月[IQR 9至30]):干预前阶段为597名,干预阶段为402名。大多数儿童(77%)由全科医生转诊,一半儿童住院。意向性治疗分析显示总体抗生素处方未减少(30%至25%,调整后的优势比[aOR] 1.07 [95%CI 0.57至2.01,p = 0.75]);策略失败率从23%降低到16%(aOR 0.53 [95%CI 0.32降至0.88,p = 0.01])。探索性分析表明,干预措施对风险人群的影响不同(p <0.01),导致低/中危儿童的抗生素处方减少(17%至6%; aOR 0.31 [95%CI 0.12至0.81,p = 0.02 ])和高风险组的显着增加(47%至59%; aOR 2.28 [95%CI 0.84至6.17,p = 0.09])。在试验过程中发生了两种并发症:随访期间1名重症监护病房住院患者和第10天1例胸膜积脓(均与研究干预无关)。该研究的主要局限性在于干预前阶段缺少CRP值,并且由于后勤问题导致基线期延长,这可能会影响我们的研究能力。结论在这项多中心ED研究中,我们观察到,儿童期肺炎的临床决策规则并未减少总体抗生素处方,但并不逊色于常规护理。探索性分析显示,在低/中危儿童中,策略失败的发生率更低,开出的抗生素更少,这表明通过决策规则可以更好地针对抗生素。试用注册荷兰试用注册号NTR5326。
更新日期:2020-02-03
中文翻译:

在荷兰,对怀疑下呼吸道感染的儿童进行抗生素处方指导的临床决策规则的评估:楔入式整群随机试验。
背景技术优化抗生素的使用是抗生素管理的关键组成部分。呼吸道感染(RTIs)是儿童服用抗生素处方的最常见原因,尽管5岁以下儿童中的大多数感染是病毒性感染。这项研究旨在通过实施临床决策规则,安全减少急诊室(ED)怀疑RTI较低的5岁以下儿童的抗生素处方。方法和研究结果在荷兰的8例ED中,我们纳入了1到60个月大的有发热,咳嗽或呼吸困难的1至60个月的儿童。急诊室规模大小不一,来自不同的地理和人口区域,并且属于不同的医院类型(三级医院与普通医院)。在干预前阶段,儿童得到了常规照料,根据针对发热儿童的荷兰语和NICE指南。在干预阶段,已实施包括临床特征和C反应蛋白(CRP)在内的经过验证的临床预测模型(Feverkidstool),作为指导抗生素处方的决策规则。干预措施是对细菌性肺炎的低或中等预测风险(≤10%,基于Feverkidstool的儿童)禁用抗生素。共同主要结果是抗生素处方率和策略失败。策略失败定义为继发抗生素处方或住院,直到第7天持续发热或依赖氧气或出现并发症。使用计算机随机化方法将医院随机分配给1种治疗顺序。审判不能盲目。我们使用多级Logistic回归来评估干预措施的效果,并按医院分组并针对时间段,年龄,性别,季节,不适,发烧持续时间进行了调整。预测风险包括在探索性分析中。我们纳入了2016年1月1日至2018年9月30日期间的999名儿童(男61%,中位年龄17个月[IQR 9至30]):干预前阶段为597名,干预阶段为402名。大多数儿童(77%)由全科医生转诊,一半儿童住院。意向性治疗分析显示总体抗生素处方未减少(30%至25%,调整后的优势比[aOR] 1.07 [95%CI 0.57至2.01,p = 0.75]);策略失败率从23%降低到16%(aOR 0.53 [95%CI 0.32降至0.88,p = 0.01])。探索性分析表明,干预措施对风险人群的影响不同(p <0.01),导致低/中危儿童的抗生素处方减少(17%至6%; aOR 0.31 [95%CI 0.12至0.81,p = 0.02 ])和高风险组的显着增加(47%至59%; aOR 2.28 [95%CI 0.84至6.17,p = 0.09])。在试验过程中发生了两种并发症:随访期间1名重症监护病房住院患者和第10天1例胸膜积脓(均与研究干预无关)。该研究的主要局限性在于干预前阶段缺少CRP值,并且由于后勤问题导致基线期延长,这可能会影响我们的研究能力。结论在这项多中心ED研究中,我们观察到,儿童期肺炎的临床决策规则并未减少总体抗生素处方,但并不逊色于常规护理。探索性分析显示,在低/中危儿童中,策略失败的发生率更低,开出的抗生素更少,这表明通过决策规则可以更好地针对抗生素。试用注册荷兰试用注册号NTR5326。











































 京公网安备 11010802027423号
京公网安备 11010802027423号