当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transthyretin Inhibits Primary and Secondary Nucleations of Amyloid-β Peptide Aggregation and Reduces the Toxicity of Its Oligomers.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-02-17 , DOI: 10.1021/acs.biomac.9b01475 Seyyed Abolghasem Ghadami 1 , Sean Chia 2 , Francesco Simone Ruggeri 2 , Georg Meisl 2 , Francesco Bemporad 1 , Johnny Habchi 2 , Roberta Cascella 1 , Christopher M Dobson 2 , Michele Vendruscolo 2 , Tuomas P J Knowles 2, 3 , Fabrizio Chiti 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-02-17 , DOI: 10.1021/acs.biomac.9b01475 Seyyed Abolghasem Ghadami 1 , Sean Chia 2 , Francesco Simone Ruggeri 2 , Georg Meisl 2 , Francesco Bemporad 1 , Johnny Habchi 2 , Roberta Cascella 1 , Christopher M Dobson 2 , Michele Vendruscolo 2 , Tuomas P J Knowles 2, 3 , Fabrizio Chiti 1
Affiliation
|
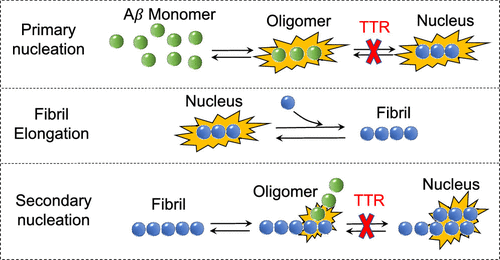
Alzheimer's disease is associated with the deposition of the amyloid-β peptide (Aβ) into extracellular senile plaques in the brain. In vitro and in vivo observations have indicated that transthyretin (TTR) acts as an Aβ scavenger in the brain, but the mechanism has not been fully resolved. We have monitored the aggregation process of Aβ40 by thioflavin T fluorescence, in the presence or absence of different concentrations of preformed seed aggregates of Aβ40, of wild-type tetrameric TTR (WT-TTR), and of a variant engineered to be stable as a monomer (M-TTR). Both WT-TTR and M-TTR were found to inhibit specific steps of the process of Aβ40 fibril formation, which are primary and secondary nucleations, without affecting the elongation of the resulting fibrils. Moreover, the analysis shows that both WT-TTR and M-TTR bind to Aβ40 oligomers formed in the aggregation reaction and inhibit their conversion into the shortest fibrils able to elongate. Using biophysical methods, TTR was found to change some aspects of its overall structure following such interactions with Aβ40 oligomers, as well as with oligomers of Aβ42, while maintaining its overall topology. Hence, it is likely that the predominant mechanism by which TTR exerts its protective role lies in the binding of TTR to the Aβ oligomers and in inhibiting primary and secondary nucleation processes, which limits both the toxicity of Aβ oligomers and the ability of the fibrils to proliferate.
中文翻译:

运甲状腺素蛋白抑制淀粉样β肽聚集的初级和次级成核,并降低其寡聚体的毒性。
阿尔茨海默氏病与淀粉样β肽(Aβ)沉积到大脑中的细胞外老年斑有关。体外和体内观察表明,运甲状腺素蛋白(TTR)在大脑中起Aβ清除剂的作用,但其机理尚未完全解决。我们已经通过硫代黄素T荧光监测了Aβ40的聚集过程,无论是否存在不同浓度的预先形成的Aβ40种子聚集体,野生型四聚体TTR(WT-TTR)以及经工程改造为稳定的变体单体(M-TTR)。发现WT-TTR和M-TTR都抑制Aβ40原纤维形成过程的特定步骤,其是初级和次级成核,而不影响所得原纤维的伸长。此外,分析表明,WT-TTR和M-TTR均与聚集反应中形成的Aβ40低聚物结合,并抑制了它们转化为能够伸长的最短原纤维。使用生物物理方法,发现TTR在与Aβ40寡聚体以及与Aβ42的寡聚体发生这种相互作用后会改变其整体结构的某些方面,同时保持其整体拓扑。因此,TTR发挥其保护作用的主要机制可能在于TTR与Aβ低聚物的结合以及抑制初级和次级成核过程,这既限制了Aβ低聚物的毒性,又限制了原纤维的能力。增生。发现TTR在与Aβ40寡聚物以及与Aβ42寡聚物相互作用之后改变其整体结构的某些方面,同时保持其整体拓扑。因此,TTR发挥其保护作用的主要机制可能在于TTR与Aβ低聚物的结合以及抑制初级和次级成核过程,这既限制了Aβ低聚物的毒性,又限制了原纤维的能力。增生。发现TTR在与Aβ40寡聚物以及与Aβ42寡聚物相互作用之后改变其整体结构的某些方面,同时保持其整体拓扑。因此,TTR发挥其保护作用的主要机制可能在于TTR与Aβ低聚物的结合以及抑制初级和次级成核过程,这既限制了Aβ低聚物的毒性,又限制了原纤维的能力。增生。
更新日期:2020-02-17
中文翻译:

运甲状腺素蛋白抑制淀粉样β肽聚集的初级和次级成核,并降低其寡聚体的毒性。
阿尔茨海默氏病与淀粉样β肽(Aβ)沉积到大脑中的细胞外老年斑有关。体外和体内观察表明,运甲状腺素蛋白(TTR)在大脑中起Aβ清除剂的作用,但其机理尚未完全解决。我们已经通过硫代黄素T荧光监测了Aβ40的聚集过程,无论是否存在不同浓度的预先形成的Aβ40种子聚集体,野生型四聚体TTR(WT-TTR)以及经工程改造为稳定的变体单体(M-TTR)。发现WT-TTR和M-TTR都抑制Aβ40原纤维形成过程的特定步骤,其是初级和次级成核,而不影响所得原纤维的伸长。此外,分析表明,WT-TTR和M-TTR均与聚集反应中形成的Aβ40低聚物结合,并抑制了它们转化为能够伸长的最短原纤维。使用生物物理方法,发现TTR在与Aβ40寡聚体以及与Aβ42的寡聚体发生这种相互作用后会改变其整体结构的某些方面,同时保持其整体拓扑。因此,TTR发挥其保护作用的主要机制可能在于TTR与Aβ低聚物的结合以及抑制初级和次级成核过程,这既限制了Aβ低聚物的毒性,又限制了原纤维的能力。增生。发现TTR在与Aβ40寡聚物以及与Aβ42寡聚物相互作用之后改变其整体结构的某些方面,同时保持其整体拓扑。因此,TTR发挥其保护作用的主要机制可能在于TTR与Aβ低聚物的结合以及抑制初级和次级成核过程,这既限制了Aβ低聚物的毒性,又限制了原纤维的能力。增生。发现TTR在与Aβ40寡聚物以及与Aβ42寡聚物相互作用之后改变其整体结构的某些方面,同时保持其整体拓扑。因此,TTR发挥其保护作用的主要机制可能在于TTR与Aβ低聚物的结合以及抑制初级和次级成核过程,这既限制了Aβ低聚物的毒性,又限制了原纤维的能力。增生。









































 京公网安备 11010802027423号
京公网安备 11010802027423号