当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Principle and design of pseudo-natural products.
Nature Chemistry ( IF 19.2 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41557-019-0411-x George Karageorgis 1, 2 , Daniel J Foley 1, 3 , Luca Laraia 1, 4 , Herbert Waldmann 1, 5
Nature Chemistry ( IF 19.2 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41557-019-0411-x George Karageorgis 1, 2 , Daniel J Foley 1, 3 , Luca Laraia 1, 4 , Herbert Waldmann 1, 5
Affiliation
|
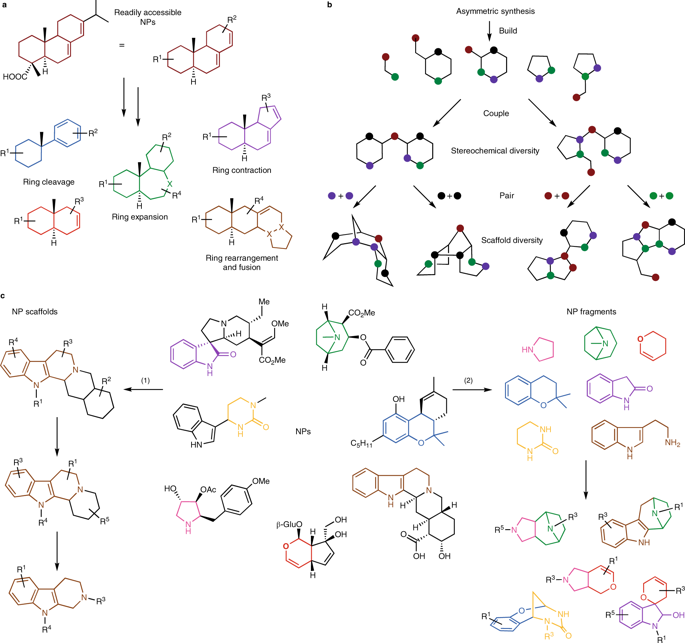
Natural products (NPs) are a significant source of inspiration towards the discovery of new bioactive compounds based on novel molecular scaffolds. However, there are currently only a small number of guiding synthetic strategies available to generate novel NP-inspired scaffolds, limiting both the number and types of compounds accessible. In this Perspective, we discuss a design approach for the preparation of biologically relevant small-molecule libraries, harnessing the unprecedented combination of NP-derived fragments as an overarching strategy for the synthesis of new bioactive compounds. These novel 'pseudo-natural product' classes retain the biological relevance of NPs, yet exhibit structures and bioactivities not accessible to nature or through the use of existing design strategies. We also analyse selected pseudo-NP libraries using chemoinformatic tools, to assess their molecular shape diversity and properties. To facilitate the exploration of biologically relevant chemical space, we identify design principles and connectivity patterns that would provide access to unprecedented pseudo-NP classes, offering new opportunities for bioactive small-molecule discovery.
中文翻译:

伪天然产品的原理与设计。
天然产物 (NPs) 是发现基于新型分子支架的新生物活性化合物的重要灵感来源。然而,目前只有少数指导性合成策略可用于生成受 NP 启发的新型支架,从而限制了可获得的化合物的数量和类型。在这个观点中,我们讨论了一种制备生物学相关小分子文库的设计方法,利用 NP 衍生片段的前所未有的组合作为合成新生物活性化合物的总体策略。这些新颖的“伪天然产物”类别保留了 NPs 的生物学相关性,但表现出自然界或通过使用现有设计策略无法获得的结构和生物活性。我们还使用化学信息学工具分析选定的伪 NP 库,以评估它们的分子形状多样性和特性。为了促进对生物相关化学空间的探索,我们确定了设计原则和连接模式,这些设计原则和连接模式将提供前所未有的伪 NP 类,为生物活性小分子的发现提供新的机会。
更新日期:2020-02-03
中文翻译:

伪天然产品的原理与设计。
天然产物 (NPs) 是发现基于新型分子支架的新生物活性化合物的重要灵感来源。然而,目前只有少数指导性合成策略可用于生成受 NP 启发的新型支架,从而限制了可获得的化合物的数量和类型。在这个观点中,我们讨论了一种制备生物学相关小分子文库的设计方法,利用 NP 衍生片段的前所未有的组合作为合成新生物活性化合物的总体策略。这些新颖的“伪天然产物”类别保留了 NPs 的生物学相关性,但表现出自然界或通过使用现有设计策略无法获得的结构和生物活性。我们还使用化学信息学工具分析选定的伪 NP 库,以评估它们的分子形状多样性和特性。为了促进对生物相关化学空间的探索,我们确定了设计原则和连接模式,这些设计原则和连接模式将提供前所未有的伪 NP 类,为生物活性小分子的发现提供新的机会。









































 京公网安备 11010802027423号
京公网安备 11010802027423号