当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Cations on Interlayer Water Dynamics in Cation-Exchanged Montmorillonites Studied by Nuclear Magnetic Resonance and X-ray Diffraction Techniques
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-02-03 , DOI: 10.1021/acsearthspacechem.9b00315 Sun Rongwei 1 , Takehiko Tsukahara 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-02-03 , DOI: 10.1021/acsearthspacechem.9b00315 Sun Rongwei 1 , Takehiko Tsukahara 1
Affiliation
|
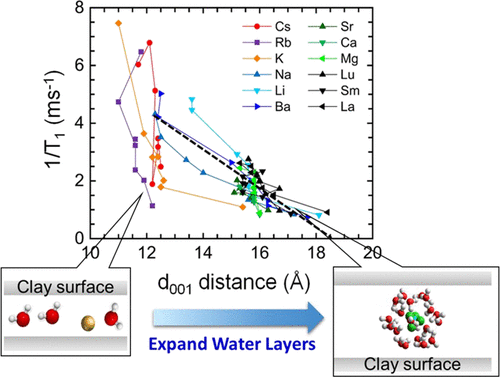
To ensure the safety of geological disposal of radioactive wastes, understanding the migration behavior of radioactive species in montmorillonite clays has become increasingly important. However, there are still many indeterminate aspects about the influence of cation species and humidity on the interlayer water dynamics and swelling properties of montmorillonite clays. In this work, by using X-ray diffraction (XRD) and proton nuclear magnetic resonance spin–lattice relaxation rate (1/T1) techniques, we aimed to clarify the relation between water layer thickness and molecular dynamics in various cation-exchanged montmorillonites (Mn+-MMTs; Mn+ = Li+, Na+, K+, Rb+, Cs+, Mg2+, Ca2+, Sr2+, Ba2+, La3+, Sm3+, and Lu3+) in the temperature range of −40 to 50 °C. XRD measurements showed that layer thickness in Mn+-MMTs increased with increasing hydrated water according to the order of structure-breaking chaotropic K+, Rb+, and Cs+ ions, structure-making kosmotropic and borderline Li+, Na+, and Ba2+ ions, divalent ions, and trivalent lanthanide ions. It was found that the 1/T1 values are linearly correlated with d001 basal spacing and increase in the interlayer thickness induces acceleration of the motion of the interlayer water molecules. The temperature dependence of the 1/T1 values verified that interparticle water and interlayer water, regardless of whether it was frozen or unfrozen, coexist above approximately −5 °C, but only unfrozen interlayer water with poor hydrogen bonding rearrangement properties exists below −5 °C. Moreover, the T1-distribution results suggest that motions of the water molecules in the Mn+-MMTs can be uniquely characterized by a value of about 0.2 ms under supercooled environments because of dominant water–surface interactions.
中文翻译:

阳离子对核磁共振和X射线衍射技术研究的阳离子交换蒙脱土层间水动力学的影响
为了确保放射性废物地质处置的安全性,了解蒙脱石粘土中放射性物质的迁移行为变得越来越重要。然而,关于阳离子种类和湿度对蒙脱石粘土层间水动力学和溶胀性的影响仍然存在许多不确定的方面。在这项工作中,通过使用X射线衍射(XRD)和质子核磁共振自旋-晶格弛豫速率(1 / T 1)技术,我们旨在阐明各种阳离子交换蒙脱土中水层厚度与分子动力学之间的关系。 (M n + -MMTs; M n + = Li +,Na +,K+,Rb +,Cs +,Mg 2 +,Ca 2 +,Sr 2 +,Ba 2 +,La 3+,Sm 3+和Lu 3+)的温度范围为-40至50°C。XRD测量结果显示以M表示层厚度Ñ + -MMTs根据结构破离液K的顺序随水合水增加+,RB +,和Cs +离子,结构制作亲液和交界李+,钠+,和钡2+离子,二价离子和三价镧系离子。已经发现1 / T 1值与d 001基间距线性相关,并且层间厚度的增加引起层间水分子运动的加速。1 / T 1值的温度依赖性证明,无论是冷冻还是未冷冻,颗粒间水和夹层水都在约-5°C以上共存,但在-5以下仅存在氢键重排性能差的未冷冻夹层水℃。此外,T 1分布结果表明,水分子在M n +中的运动-MMTs的独特特征是在过冷环境下,由于主要的水-地相互作用,其值约为0.2 ms。
更新日期:2020-02-03
中文翻译:

阳离子对核磁共振和X射线衍射技术研究的阳离子交换蒙脱土层间水动力学的影响
为了确保放射性废物地质处置的安全性,了解蒙脱石粘土中放射性物质的迁移行为变得越来越重要。然而,关于阳离子种类和湿度对蒙脱石粘土层间水动力学和溶胀性的影响仍然存在许多不确定的方面。在这项工作中,通过使用X射线衍射(XRD)和质子核磁共振自旋-晶格弛豫速率(1 / T 1)技术,我们旨在阐明各种阳离子交换蒙脱土中水层厚度与分子动力学之间的关系。 (M n + -MMTs; M n + = Li +,Na +,K+,Rb +,Cs +,Mg 2 +,Ca 2 +,Sr 2 +,Ba 2 +,La 3+,Sm 3+和Lu 3+)的温度范围为-40至50°C。XRD测量结果显示以M表示层厚度Ñ + -MMTs根据结构破离液K的顺序随水合水增加+,RB +,和Cs +离子,结构制作亲液和交界李+,钠+,和钡2+离子,二价离子和三价镧系离子。已经发现1 / T 1值与d 001基间距线性相关,并且层间厚度的增加引起层间水分子运动的加速。1 / T 1值的温度依赖性证明,无论是冷冻还是未冷冻,颗粒间水和夹层水都在约-5°C以上共存,但在-5以下仅存在氢键重排性能差的未冷冻夹层水℃。此外,T 1分布结果表明,水分子在M n +中的运动-MMTs的独特特征是在过冷环境下,由于主要的水-地相互作用,其值约为0.2 ms。











































 京公网安备 11010802027423号
京公网安备 11010802027423号