当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The reaction of arylmethyl isocyanides and arylmethylamines with xanthate esters: a facile and unexpected synthesis of carbamothioates.
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-02-03 , DOI: 10.3762/bjoc.16.18 Narasimhamurthy Rajeev 1 , Toreshettahally R Swaroop 1, 2 , Ahmad I Alrawashdeh 2 , Shofiur Rahman 2, 3 , Abdullah Alodhayb 3, 4 , Seegehalli M Anil 1 , Kuppalli R Kiran 1 , Chandra 5 , Paris E Georghiou 2 , Kanchugarakoppal S Rangappa 1 , Maralinganadoddi P Sadashiva 1
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-02-03 , DOI: 10.3762/bjoc.16.18 Narasimhamurthy Rajeev 1 , Toreshettahally R Swaroop 1, 2 , Ahmad I Alrawashdeh 2 , Shofiur Rahman 2, 3 , Abdullah Alodhayb 3, 4 , Seegehalli M Anil 1 , Kuppalli R Kiran 1 , Chandra 5 , Paris E Georghiou 2 , Kanchugarakoppal S Rangappa 1 , Maralinganadoddi P Sadashiva 1
Affiliation
|
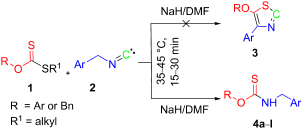
An unexpected formation of carbamothioates by a sodium hydride-mediated reaction of arylmethyl isocyanides with xanthate esters in DMF is reported. The products thus obtained were compared with the carbamothioates obtained by the sodium hydride-mediated condensation of the corresponding benzylamines and xanthate esters in DMF. To account for these unexpected reactions, a mechanism is proposed in which the key steps are supported by quantum chemical calculations.
中文翻译:

芳基甲基异氰化物和芳基甲基胺与黄原酸酯的反应:氨基甲酸酯的便捷合成。
据报道,在DMF中,氢化钠介导的芳基甲基异氰化物与黄原酸酯的反应会意外生成氨基甲酸酯。将由此获得的产物与通过氢化钠介导的相应的苄胺和黄原酸酯在DMF中的氢化钠介导的缩合而获得的氨基甲酸酯进行比较。为了解决这些意外反应,提出了一种机制,其中关键步骤得到了量子化学计算的支持。
更新日期:2020-02-03
中文翻译:

芳基甲基异氰化物和芳基甲基胺与黄原酸酯的反应:氨基甲酸酯的便捷合成。
据报道,在DMF中,氢化钠介导的芳基甲基异氰化物与黄原酸酯的反应会意外生成氨基甲酸酯。将由此获得的产物与通过氢化钠介导的相应的苄胺和黄原酸酯在DMF中的氢化钠介导的缩合而获得的氨基甲酸酯进行比较。为了解决这些意外反应,提出了一种机制,其中关键步骤得到了量子化学计算的支持。



























 京公网安备 11010802027423号
京公网安备 11010802027423号