当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An investigation into the precipitation of copper sulfide from acidic sulfate solutions
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.hydromet.2020.105288 Tao Hong , Yan Wei , Linbo Li , Kathryn A. Mumford , Geoffrey W. Stevens
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.hydromet.2020.105288 Tao Hong , Yan Wei , Linbo Li , Kathryn A. Mumford , Geoffrey W. Stevens
|
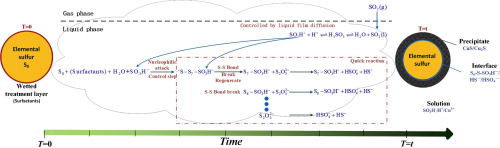
Abstract Due to the local and global supersaturation of metal sulfides in aqueous solutions, the industrial metal sulfurization processes using soluble sulfur resources (Na2S/Na2S2O3/(NH4)2S/H2S) and elemental sulfur have insurmountable defects, including the unfilterable fine precipitates, weak chemical selectivity, high reagent toxicity, and generation of soluble polysulfides. In order to develop a cleaner and green sulfurization method, a surface/heterogeneous sulfurization system using wetted sulfur particles and sulfur dioxide was investigated through single-factor experiments and reaction mechanism tests. The results of disproportionation tests indicated that reaction parameters, such as temperature, powder contact angle, SO2 pressure and molar ratio of Cu-to-Sulfur, significantly affected the reaction efficiency. The reaction is mixed controlled by the temperature and powder contact angle. During the disproportionation‑sulfurization process, the total reaction rate for the generation of HS− ions is dependent upon: the liquid diffusion of SO2 absorption, the powder contact angle of wetted sulfur particles, and the nucleophilic reaction between SO3H− with sulfur. Meanwhile, the total reaction is a shrinking nuclear reaction which the particles sizes continuously increasing after the metal sulfides formed.
中文翻译:

酸性硫酸盐溶液中硫化铜沉淀的研究
摘要 由于水溶液中金属硫化物的局部和全局过饱和,利用可溶性硫资源(Na2S/Na2S2O3/(NH4)2S/H2S)和元素硫的工业金属硫化工艺存在无法克服的缺陷,包括无法过滤的细小沉淀物、弱化学选择性、高试剂毒性和可溶性多硫化物的生成。为了开发更清洁、绿色的硫化方法,通过单因素实验和反应机理测试,研究了使用湿硫颗粒和二氧化硫的表面/非均相硫化系统。歧化试验结果表明,反应参数,如温度、粉末接触角、SO2 压力和铜硫摩尔比对反应效率有显着影响。通过温度和粉末接触角控制反应混合。在歧化-硫化过程中,生成 HS− 离子的总反应速率取决于:SO2 吸收的液体扩散、湿硫颗粒的粉末接触角以及 SO3H− 与硫之间的亲核反应。同时,整个反应是一个收缩核反应,金属硫化物形成后颗粒尺寸不断增大。
更新日期:2020-03-01
中文翻译:

酸性硫酸盐溶液中硫化铜沉淀的研究
摘要 由于水溶液中金属硫化物的局部和全局过饱和,利用可溶性硫资源(Na2S/Na2S2O3/(NH4)2S/H2S)和元素硫的工业金属硫化工艺存在无法克服的缺陷,包括无法过滤的细小沉淀物、弱化学选择性、高试剂毒性和可溶性多硫化物的生成。为了开发更清洁、绿色的硫化方法,通过单因素实验和反应机理测试,研究了使用湿硫颗粒和二氧化硫的表面/非均相硫化系统。歧化试验结果表明,反应参数,如温度、粉末接触角、SO2 压力和铜硫摩尔比对反应效率有显着影响。通过温度和粉末接触角控制反应混合。在歧化-硫化过程中,生成 HS− 离子的总反应速率取决于:SO2 吸收的液体扩散、湿硫颗粒的粉末接触角以及 SO3H− 与硫之间的亲核反应。同时,整个反应是一个收缩核反应,金属硫化物形成后颗粒尺寸不断增大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号