当前位置:
X-MOL 学术
›
Nat. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Designing ecologically optimized pneumococcal vaccines using population genomics.
Nature Microbiology ( IF 20.5 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41564-019-0651-y Caroline Colijn 1, 2 , Jukka Corander 3, 4, 5 , Nicholas J Croucher 6
Nature Microbiology ( IF 20.5 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41564-019-0651-y Caroline Colijn 1, 2 , Jukka Corander 3, 4, 5 , Nicholas J Croucher 6
Affiliation
|
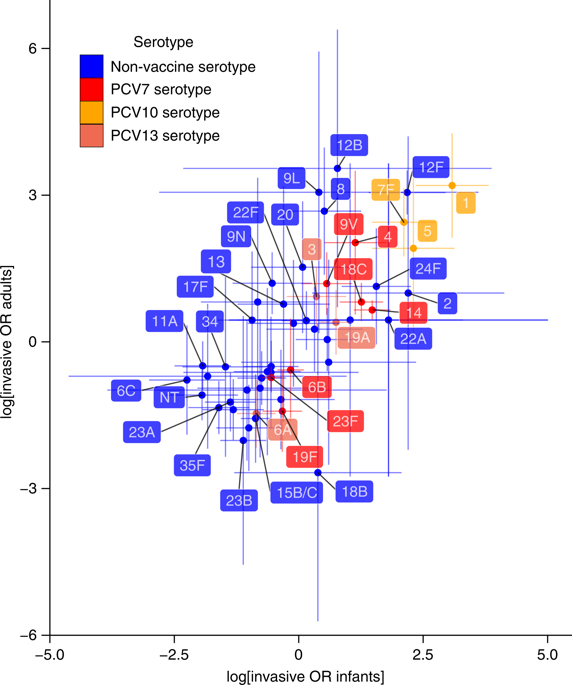
Streptococcus pneumoniae (the pneumococcus) is a common nasopharyngeal commensal that can cause invasive pneumococcal disease (IPD). Each component of current protein-polysaccharide conjugate vaccines (PCVs) generally induces immunity specific to one of the approximately 100 pneumococcal serotypes, and typically eliminates it from carriage and IPD through herd immunity. Overall carriage rates remain stable owing to replacement by non-PCV serotypes. Consequently, the net change in IPD incidence is determined by the relative invasiveness of the pre- and post-PCV-carried pneumococcal populations. In the present study, we identified PCVs expected to minimize the post-vaccine IPD burden by applying Bayesian optimization to an ecological model of serotype replacement that integrated epidemiological and genomic data. We compared optimal formulations for reducing infant-only or population-wide IPD, and identified potential benefits to including non-conserved pneumococcal carrier proteins. Vaccines were also devised to minimize IPD resistant to antibiotic treatment, despite the ecological model assuming that resistance levels in the carried population would be preserved. We found that expanding infant-administered PCV valency is likely to result in diminishing returns, and that complementary pairs of infant- and adult-administered vaccines could be a superior strategy. PCV performance was highly dependent on the circulating pneumococcal population, further highlighting the advantages of a diversity of anti-pneumococcal vaccination strategies.
中文翻译:

利用群体基因组学设计生态优化的肺炎球菌疫苗。
肺炎链球菌(肺炎球菌)是一种常见的鼻咽共生菌,可引起侵袭性肺炎球菌疾病 (IPD)。目前的蛋白质-多糖结合疫苗 (PCV) 的每种成分通常都会诱导针对大约 100 种肺炎球菌血清型中的一种的特异性免疫,并通常通过群体免疫将其从携带和 IPD 中消除。由于被非 PCV 血清型替代,总体携带率保持稳定。因此,IPD 发病率的净变化取决于 PCV 携带前和携带后肺炎球菌群体的相对侵袭性。在本研究中,我们通过将贝叶斯优化应用于整合流行病学和基因组数据的血清型替代生态模型,确定了有望最大限度减少疫苗后 IPD 负担的 PCV。我们比较了减少婴儿或全人群 IPD 的最佳配方,并确定了包含非保守肺炎球菌载体蛋白的潜在益处。尽管生态模型假设携带人群的耐药水平将保持不变,但疫苗的设计也是为了最大限度地减少 IPD 对抗生素治疗的耐药性。我们发现,扩大婴儿接种 PCV 效价可能会导致收益递减,而婴儿和成人接种疫苗的互补配对可能是更好的策略。PCV 性能高度依赖于循环肺炎球菌群,进一步凸显了多种抗肺炎球菌疫苗接种策略的优势。
更新日期:2020-02-03
中文翻译:

利用群体基因组学设计生态优化的肺炎球菌疫苗。
肺炎链球菌(肺炎球菌)是一种常见的鼻咽共生菌,可引起侵袭性肺炎球菌疾病 (IPD)。目前的蛋白质-多糖结合疫苗 (PCV) 的每种成分通常都会诱导针对大约 100 种肺炎球菌血清型中的一种的特异性免疫,并通常通过群体免疫将其从携带和 IPD 中消除。由于被非 PCV 血清型替代,总体携带率保持稳定。因此,IPD 发病率的净变化取决于 PCV 携带前和携带后肺炎球菌群体的相对侵袭性。在本研究中,我们通过将贝叶斯优化应用于整合流行病学和基因组数据的血清型替代生态模型,确定了有望最大限度减少疫苗后 IPD 负担的 PCV。我们比较了减少婴儿或全人群 IPD 的最佳配方,并确定了包含非保守肺炎球菌载体蛋白的潜在益处。尽管生态模型假设携带人群的耐药水平将保持不变,但疫苗的设计也是为了最大限度地减少 IPD 对抗生素治疗的耐药性。我们发现,扩大婴儿接种 PCV 效价可能会导致收益递减,而婴儿和成人接种疫苗的互补配对可能是更好的策略。PCV 性能高度依赖于循环肺炎球菌群,进一步凸显了多种抗肺炎球菌疫苗接种策略的优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号