当前位置:
X-MOL 学术
›
Nat. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A myelin-related transcriptomic profile is shared by Pitt-Hopkins syndrome models and human autism spectrum disorder.
Nature Neuroscience ( IF 21.2 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41593-019-0578-x BaDoi N Phan 1, 2 , Joseph F Bohlen 1 , Brittany A Davis 1 , Zengyou Ye 1 , Huei-Ying Chen 1 , Brent Mayfield 1 , Srinidhi Rao Sripathy 1 , Stephanie Cerceo Page 1 , Morganne N Campbell 1 , Hannah L Smith 1 , Danisha Gallop 1 , Hyojin Kim 3, 4 , Courtney L Thaxton 3, 4 , Jeremy M Simon 3, 5, 6 , Emily E Burke 1 , Joo Heon Shin 1, 7 , Andrew J Kennedy 8 , J David Sweatt 9 , Benjamin D Philpot 3, 4, 6 , Andrew E Jaffe 1, 10, 11, 12, 13, 14 , Brady J Maher 1, 12, 14
Nature Neuroscience ( IF 21.2 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41593-019-0578-x BaDoi N Phan 1, 2 , Joseph F Bohlen 1 , Brittany A Davis 1 , Zengyou Ye 1 , Huei-Ying Chen 1 , Brent Mayfield 1 , Srinidhi Rao Sripathy 1 , Stephanie Cerceo Page 1 , Morganne N Campbell 1 , Hannah L Smith 1 , Danisha Gallop 1 , Hyojin Kim 3, 4 , Courtney L Thaxton 3, 4 , Jeremy M Simon 3, 5, 6 , Emily E Burke 1 , Joo Heon Shin 1, 7 , Andrew J Kennedy 8 , J David Sweatt 9 , Benjamin D Philpot 3, 4, 6 , Andrew E Jaffe 1, 10, 11, 12, 13, 14 , Brady J Maher 1, 12, 14
Affiliation
|
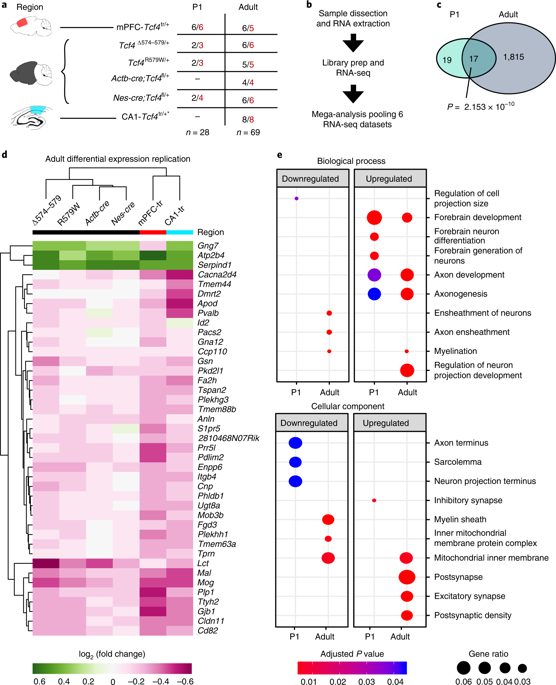
Autism spectrum disorder (ASD) is genetically heterogeneous with convergent symptomatology, suggesting common dysregulated pathways. In this study, we analyzed brain transcriptional changes in five mouse models of Pitt-Hopkins syndrome (PTHS), a syndromic form of ASD caused by mutations in the TCF4 gene, but not the TCF7L2 gene. Analyses of differentially expressed genes (DEGs) highlighted oligodendrocyte (OL) dysregulation, which we confirmed in two additional mouse models of syndromic ASD (Ptenm3m4/m3m4 and Mecp2tm1.1Bird). The PTHS mouse models showed cell-autonomous reductions in OL numbers and myelination, functionally confirming OL transcriptional signatures. We also integrated PTHS mouse model DEGs with human idiopathic ASD postmortem brain RNA-sequencing data and found significant enrichment of overlapping DEGs and common myelination-associated pathways. Notably, DEGs from syndromic ASD mouse models and reduced deconvoluted OL numbers distinguished human idiopathic ASD cases from controls across three postmortem brain data sets. These results implicate disruptions in OL biology as a cellular mechanism in ASD pathology.
中文翻译:

Pitt-Hopkins综合征模型和人类自闭症谱系障碍共享髓鞘相关的转录组谱。
自闭症谱系障碍(ASD)是遗传异质性的,具有收敛的症状,提示常见的失调途径。在这项研究中,我们分析了Pitt-Hopkins综合征(PTHS)的五种小鼠模型中的脑转录变化,这是一种TSD的症状形式,由TCF4基因突变引起,而不是TCF7L2基因突变。差异表达基因(DEGs)的分析突出了少突胶质细胞(OL)的失调,我们在另外两种具有综合征性ASD的小鼠模型(Ptenm3m4 / m3m4和Mecp2tm1.1Bird)中证实了这一点。PTHS小鼠模型显示OL数量和髓鞘形成的细胞自主减少,在功能上证实了OL转录特征。我们还将PTHS小鼠模型DEG与人类特发性ASD死后脑RNA测序数据进行了整合,发现重叠DEG和常见的髓鞘相关途径显着富集。值得注意的是,来自有症状的ASD小鼠模型的DEG和减少的去卷积OL数量将人类特发性ASD病例与三个死后大脑数据集中的对照区分开来。这些结果暗示了作为ASD病理学中一种细胞机制的OL生物学的破坏。
更新日期:2020-02-03
中文翻译:

Pitt-Hopkins综合征模型和人类自闭症谱系障碍共享髓鞘相关的转录组谱。
自闭症谱系障碍(ASD)是遗传异质性的,具有收敛的症状,提示常见的失调途径。在这项研究中,我们分析了Pitt-Hopkins综合征(PTHS)的五种小鼠模型中的脑转录变化,这是一种TSD的症状形式,由TCF4基因突变引起,而不是TCF7L2基因突变。差异表达基因(DEGs)的分析突出了少突胶质细胞(OL)的失调,我们在另外两种具有综合征性ASD的小鼠模型(Ptenm3m4 / m3m4和Mecp2tm1.1Bird)中证实了这一点。PTHS小鼠模型显示OL数量和髓鞘形成的细胞自主减少,在功能上证实了OL转录特征。我们还将PTHS小鼠模型DEG与人类特发性ASD死后脑RNA测序数据进行了整合,发现重叠DEG和常见的髓鞘相关途径显着富集。值得注意的是,来自有症状的ASD小鼠模型的DEG和减少的去卷积OL数量将人类特发性ASD病例与三个死后大脑数据集中的对照区分开来。这些结果暗示了作为ASD病理学中一种细胞机制的OL生物学的破坏。











































 京公网安备 11010802027423号
京公网安备 11010802027423号