当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein-Induced Change in Ligand Protonation during Trypsin and Thrombin Binding: Hint on Differences in Selectivity Determinants of Both Proteins?
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-02-24 , DOI: 10.1021/acs.jmedchem.9b02061 Khang Ngo 1 , Chelsey Collins-Kautz 1 , Stefan Gerstenecker 1 , Björn Wagner 2 , Andreas Heine 1 , Gerhard Klebe 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-02-24 , DOI: 10.1021/acs.jmedchem.9b02061 Khang Ngo 1 , Chelsey Collins-Kautz 1 , Stefan Gerstenecker 1 , Björn Wagner 2 , Andreas Heine 1 , Gerhard Klebe 1
Affiliation
|
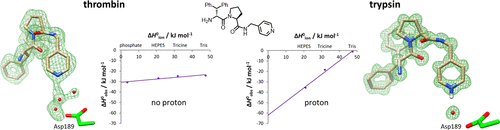
Trypsin and thrombin, structurally similar serine proteases, recognize different substrates; thrombin cleaves after Arg, whereas trypsin cleaves after Lys/Arg. Both recognize basic substrate headgroups via Asp189 at the bottom of the S1 pocket. By crystallography and isothermal titration calorimetry (ITC), we studied a series of d-Phe/d-DiPhe-Pro-(amino)pyridines. Identical ligand pairs show the same binding poses. Surprisingly, one ligand binds to trypsin in protonated state and to thrombin in unprotonated state at P1 along with differences in the residual solvation pattern. While trypsin binding is mediated by an ordered water molecule, in thrombin, water is scattered over three hydration sites. Although having highly similar S1 pockets, our results suggest different electrostatic properties of Asp189 possibly contributing to the selectivity determinant. Thrombin binds a specific Na+ ion next to Asp189, which is absent in trypsin. The electrostatic properties across the S1 pocket are further attenuated by charged Glu192 at the rim of S1 in thrombin, which is replaced by uncharged Gln192 in trypsin.
中文翻译:

胰蛋白酶和凝血酶结合过程中蛋白质诱导的配体质子化变化:是否暗示两种蛋白质的选择性决定因素不同?
胰蛋白酶和凝血酶是结构相似的丝氨酸蛋白酶,可识别不同的底物。凝血酶在Arg后裂解,而胰蛋白酶在Lys / Arg后裂解。两者都通过S1口袋底部的Asp189识别基本的基板头基。通过晶体学和等温滴定热法(ITC),我们研究了一系列d -Phe / d-DiPhe-Pro-(氨基)吡啶。相同的配体对显示相同的结合姿势。出人意料的是,一种配体在P1处以质子化状态的胰蛋白酶和未质子化状态的凝血酶结合,并残留溶剂化方式不同。虽然胰蛋白酶结合是由有序的水分子介导的,但在凝血酶中,水却分散在三个水合位点上。尽管具有高度相似的S1口袋,我们的结果表明Asp189的不同静电性质可能有助于选择性的决定因素。凝血酶与胰蛋白酶中不存在的Asp189旁的特定Na +离子结合。凝血酶中S1边缘的带电Glu192进一步削弱了整个S1袋的静电特性,而胰蛋白酶中的不带电Gln192代替了该电荷。
更新日期:2020-02-24
中文翻译:

胰蛋白酶和凝血酶结合过程中蛋白质诱导的配体质子化变化:是否暗示两种蛋白质的选择性决定因素不同?
胰蛋白酶和凝血酶是结构相似的丝氨酸蛋白酶,可识别不同的底物。凝血酶在Arg后裂解,而胰蛋白酶在Lys / Arg后裂解。两者都通过S1口袋底部的Asp189识别基本的基板头基。通过晶体学和等温滴定热法(ITC),我们研究了一系列d -Phe / d-DiPhe-Pro-(氨基)吡啶。相同的配体对显示相同的结合姿势。出人意料的是,一种配体在P1处以质子化状态的胰蛋白酶和未质子化状态的凝血酶结合,并残留溶剂化方式不同。虽然胰蛋白酶结合是由有序的水分子介导的,但在凝血酶中,水却分散在三个水合位点上。尽管具有高度相似的S1口袋,我们的结果表明Asp189的不同静电性质可能有助于选择性的决定因素。凝血酶与胰蛋白酶中不存在的Asp189旁的特定Na +离子结合。凝血酶中S1边缘的带电Glu192进一步削弱了整个S1袋的静电特性,而胰蛋白酶中的不带电Gln192代替了该电荷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号