当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Macrocyclic Inhibitors of Human Cathepsin D: Structure-Activity Relationship and Binding Mode Analysis.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-02-13 , DOI: 10.1021/acs.jmedchem.9b01351 Radka Houštecká 1, 2 , Martin Hadzima 1, 3 , Jindřich Fanfrlík 1 , Jiří Brynda 1 , Lenka Pallová 1 , Iva Hánová 1, 4 , Helena Mertlíková-Kaiserová 1 , Martin Lepšík 1 , Martin Horn 1 , Martin Smrčina 5 , Pavel Majer 1 , Michael Mareš 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-02-13 , DOI: 10.1021/acs.jmedchem.9b01351 Radka Houštecká 1, 2 , Martin Hadzima 1, 3 , Jindřich Fanfrlík 1 , Jiří Brynda 1 , Lenka Pallová 1 , Iva Hánová 1, 4 , Helena Mertlíková-Kaiserová 1 , Martin Lepšík 1 , Martin Horn 1 , Martin Smrčina 5 , Pavel Majer 1 , Michael Mareš 1
Affiliation
|
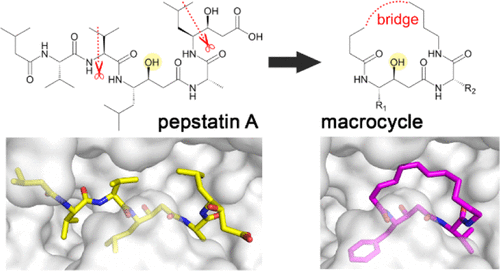
Human cathepsin D (CatD), a pepsin-family aspartic protease, plays an important role in tumor progression and metastasis. Here, we report the development of biomimetic inhibitors of CatD as novel tools for regulation of this therapeutic target. We designed a macrocyclic scaffold to mimic the spatial conformation of the minimal pseudo-dipeptide binding motif of pepstatin A, a microbial oligopeptide inhibitor, in the CatD active site. A library of more than 30 macrocyclic peptidomimetic inhibitors was employed for scaffold optimization, mapping of subsite interactions, and profiling of inhibitor selectivity. Furthermore, we solved high-resolution crystal structures of three macrocyclic inhibitors with low nanomolar or subnanomolar potency in complex with CatD and determined their binding mode using quantum chemical calculations. The study provides a new structural template and functional profile that can be exploited for design of potential chemotherapeutics that specifically inhibit CatD and related aspartic proteases.
中文翻译:

人类组织蛋白酶D的仿生大环抑制剂:结构-活性关系和结合模式分析。
人组织蛋白酶D(CatD)是一种胃蛋白酶家族的天冬氨酸蛋白酶,在肿瘤的进展和转移中起着重要的作用。在这里,我们报告了仿生抑制剂CatD的发展,作为调节该治疗靶标的新型工具。我们设计了一个大环支架来模拟CatD活性位点中抑肽酶抑素A(一种微生物寡肽抑制剂)的最小假二肽结合基序的空间构象。使用30多种大环拟肽抑制剂的库进行支架优化,亚位点相互作用的作图以及抑制剂选择性的分析。此外,我们与CatD配合使用了三种具有低纳摩尔或亚纳摩尔能力的大环抑制剂的高分辨率晶体结构,并通过量子化学计算确定了它们的结合模式。
更新日期:2020-02-13
中文翻译:

人类组织蛋白酶D的仿生大环抑制剂:结构-活性关系和结合模式分析。
人组织蛋白酶D(CatD)是一种胃蛋白酶家族的天冬氨酸蛋白酶,在肿瘤的进展和转移中起着重要的作用。在这里,我们报告了仿生抑制剂CatD的发展,作为调节该治疗靶标的新型工具。我们设计了一个大环支架来模拟CatD活性位点中抑肽酶抑素A(一种微生物寡肽抑制剂)的最小假二肽结合基序的空间构象。使用30多种大环拟肽抑制剂的库进行支架优化,亚位点相互作用的作图以及抑制剂选择性的分析。此外,我们与CatD配合使用了三种具有低纳摩尔或亚纳摩尔能力的大环抑制剂的高分辨率晶体结构,并通过量子化学计算确定了它们的结合模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号