当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A phase 2 study of intraperitoneal carboplatin plus intravenous dose-dense paclitaxel in front-line treatment of suboptimal residual ovarian cancer.
British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-01-31 , DOI: 10.1038/s41416-020-0734-9 Kosei Hasegawa 1, 2 , Muneaki Shimada 3 , Satoshi Takeuchi 4 , Hiroyuki Fujiwara 5 , Yuichi Imai 1 , Norihiro Iwasa 1, 2 , Satoru Wada 2 , Hidetaka Eguchi 2, 6 , Tetsuro Oishi 3 , Toru Sugiyama 4 , Mitsuaki Suzuki 5 , Masahiko Nishiyama 2, 6, 7 , Keiichi Fujiwara 1, 2
British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-01-31 , DOI: 10.1038/s41416-020-0734-9 Kosei Hasegawa 1, 2 , Muneaki Shimada 3 , Satoshi Takeuchi 4 , Hiroyuki Fujiwara 5 , Yuichi Imai 1 , Norihiro Iwasa 1, 2 , Satoru Wada 2 , Hidetaka Eguchi 2, 6 , Tetsuro Oishi 3 , Toru Sugiyama 4 , Mitsuaki Suzuki 5 , Masahiko Nishiyama 2, 6, 7 , Keiichi Fujiwara 1, 2
Affiliation
|
BACKGROUND
We evaluated the efficacy of intraperitoneal (IP) carboplatin in combination with dose-dense paclitaxel (ddTCip) for suboptimal residual ovarian cancer.
METHODS
This was a phase 2 study to evaluate ddTCip. Patients with stage II-IV ovarian carcinoma, who underwent primary cytoreductive surgery and had radiologically evaluable disease after surgery, were eligible to participate in this study. IP carboplatin (AUC = 6) was administered on day 1, and intravenous paclitaxel (80 mg/m2) was administered on days 1, 8 and 15. The primary endpoint was response rate. Secondary endpoints included progression-free survival (PFS), overall survival (OS) and safety. Interval- debulking surgery followed by the same regimen was allowed when indicated.
RESULTS
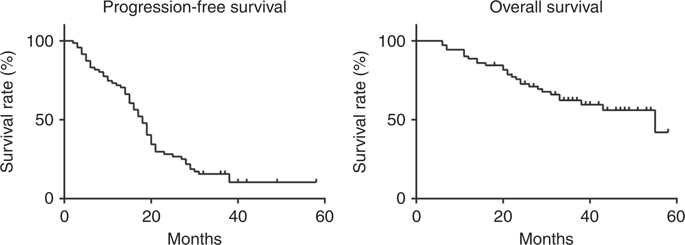
A total of 117 patients were considered eligible for this study prior to surgery and temporarily registered. Of the 117 patients, 76 patients met the inclusion criteria and were enrolled in this study. Fifty-nine (83.1%) patients had objective clinical responses. Median PFS and OS were 18.3 and 55.5 months, respectively. Sixty-four (84.2%) patients had grade 3/4 neutropenia, 43 (56.5%) patients had anaemia and 17 (22.4%) patients had thrombocytopenia. Port-related adverse events occurred in nine (11.8%) patients.
CONCLUSIONS
Front-line chemotherapy with ddTCip therapy appears safe and effective, even for patients with suboptimal residual ovarian cancer.
TRIAL REGISTRATION
UMIN Clinical Trials Registry (ID: UMIN000001713) on February 16th, 2009.
中文翻译:

腹腔内卡铂加静脉剂量密集紫杉醇一线治疗次优残留卵巢癌的 2 期研究。
背景我们评估了腹腔内(IP)卡铂联合剂量密集型紫杉醇(ddTCip)治疗次优残留卵巢癌的疗效。方法 这是一项评估 ddTCip 的 2 期研究。接受初次细胞减灭术且术后具有放射学可评估疾病的 II-IV 期卵巢癌患者有资格参加本研究。第 1 天静脉注射卡铂 (AUC = 6),第 1、8 和 15 天静脉注射紫杉醇 (80 mg/m2)。主要终点是缓解率。次要终点包括无进展生存期(PFS)、总生存期(OS)和安全性。当有指征时,允许进行间隔减瘤手术,然后进行相同的治疗方案。结果 共有 117 名患者在手术前被认为有资格参加本研究并暂时登记。在 117 名患者中,76 名患者符合纳入标准并参加了本研究。 59 名 (83.1%) 患者有客观的临床反应。中位 PFS 和 OS 分别为 18.3 个月和 55.5 个月。 64 名患者 (84.2%) 患有 3/4 级中性粒细胞减少症,43 名患者 (56.5%) 患有贫血,17 名患者 (22.4%) 患有血小板减少症。九名 (11.8%) 患者发生了与端口相关的不良事件。结论 使用 ddTCip 疗法进行一线化疗似乎是安全有效的,即使对于残留卵巢癌状况不佳的患者也是如此。试验注册 UMIN 临床试验注册中心(ID:UMIN000001713),2009 年 2 月 16 日。
更新日期:2020-01-31
中文翻译:

腹腔内卡铂加静脉剂量密集紫杉醇一线治疗次优残留卵巢癌的 2 期研究。
背景我们评估了腹腔内(IP)卡铂联合剂量密集型紫杉醇(ddTCip)治疗次优残留卵巢癌的疗效。方法 这是一项评估 ddTCip 的 2 期研究。接受初次细胞减灭术且术后具有放射学可评估疾病的 II-IV 期卵巢癌患者有资格参加本研究。第 1 天静脉注射卡铂 (AUC = 6),第 1、8 和 15 天静脉注射紫杉醇 (80 mg/m2)。主要终点是缓解率。次要终点包括无进展生存期(PFS)、总生存期(OS)和安全性。当有指征时,允许进行间隔减瘤手术,然后进行相同的治疗方案。结果 共有 117 名患者在手术前被认为有资格参加本研究并暂时登记。在 117 名患者中,76 名患者符合纳入标准并参加了本研究。 59 名 (83.1%) 患者有客观的临床反应。中位 PFS 和 OS 分别为 18.3 个月和 55.5 个月。 64 名患者 (84.2%) 患有 3/4 级中性粒细胞减少症,43 名患者 (56.5%) 患有贫血,17 名患者 (22.4%) 患有血小板减少症。九名 (11.8%) 患者发生了与端口相关的不良事件。结论 使用 ddTCip 疗法进行一线化疗似乎是安全有效的,即使对于残留卵巢癌状况不佳的患者也是如此。试验注册 UMIN 临床试验注册中心(ID:UMIN000001713),2009 年 2 月 16 日。











































 京公网安备 11010802027423号
京公网安备 11010802027423号