当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron Transfer in Nitrogenase.
Chemical Reviews ( IF 51.4 ) Pub Date : 2020-01-30 , DOI: 10.1021/acs.chemrev.9b00663 Hannah L Rutledge 1 , F Akif Tezcan 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2020-01-30 , DOI: 10.1021/acs.chemrev.9b00663 Hannah L Rutledge 1 , F Akif Tezcan 1
Affiliation
|
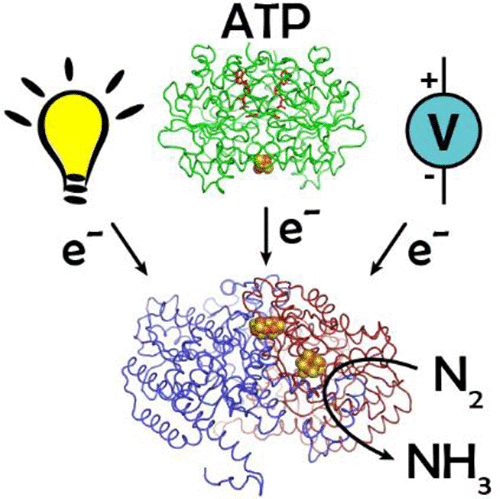
Nitrogenase is the only enzyme capable of reducing N2 to NH3. This challenging reaction requires the coordinated transfer of multiple electrons from the reductase, Fe-protein, to the catalytic component, MoFe-protein, in an ATP-dependent fashion. In the last two decades, there have been significant advances in our understanding of how nitrogenase orchestrates electron transfer (ET) from the Fe-protein to the catalytic site of MoFe-protein and how energy from ATP hydrolysis transduces the ET processes. In this review, we summarize these advances, with focus on the structural and thermodynamic redox properties of nitrogenase component proteins and their complexes, as well as on new insights regarding the mechanism of ET reactions during catalysis and how they are coupled to ATP hydrolysis. We also discuss recently developed chemical, photochemical, and electrochemical methods for uncoupling substrate reduction from ATP hydrolysis, which may provide new avenues for studying the catalytic mechanism of nitrogenase.
中文翻译:

固氮酶中的电子转移。
固氮酶是唯一能够将N 2还原为NH 3 的酶。这一具有挑战性的反应需要多个电子以 ATP 依赖性方式从还原酶(Fe 蛋白)协调转移到催化成分(MoFe 蛋白)。在过去的二十年中,我们对固氮酶如何协调从 Fe 蛋白到 MoFe 蛋白催化位点的电子转移 (ET) 以及 ATP 水解产生的能量如何转导 ET 过程的理解取得了重大进展。在这篇综述中,我们总结了这些进展,重点关注固氮酶成分蛋白及其复合物的结构和热力学氧化还原特性,以及关于催化过程中 ET 反应机制以及它们如何与 ATP 水解耦合的新见解。我们还讨论了最近开发的化学、光化学和电化学方法,用于解偶联 ATP 水解的底物还原,这可能为研究固氮酶的催化机制提供新的途径。
更新日期:2020-01-30
中文翻译:

固氮酶中的电子转移。
固氮酶是唯一能够将N 2还原为NH 3 的酶。这一具有挑战性的反应需要多个电子以 ATP 依赖性方式从还原酶(Fe 蛋白)协调转移到催化成分(MoFe 蛋白)。在过去的二十年中,我们对固氮酶如何协调从 Fe 蛋白到 MoFe 蛋白催化位点的电子转移 (ET) 以及 ATP 水解产生的能量如何转导 ET 过程的理解取得了重大进展。在这篇综述中,我们总结了这些进展,重点关注固氮酶成分蛋白及其复合物的结构和热力学氧化还原特性,以及关于催化过程中 ET 反应机制以及它们如何与 ATP 水解耦合的新见解。我们还讨论了最近开发的化学、光化学和电化学方法,用于解偶联 ATP 水解的底物还原,这可能为研究固氮酶的催化机制提供新的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号