当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Lignin Depolymerization to Aromatic Chemicals.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-01-30 , DOI: 10.1021/acs.accounts.9b00573 Chaofeng Zhang 1 , Feng Wang 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-01-30 , DOI: 10.1021/acs.accounts.9b00573 Chaofeng Zhang 1 , Feng Wang 1
Affiliation
|
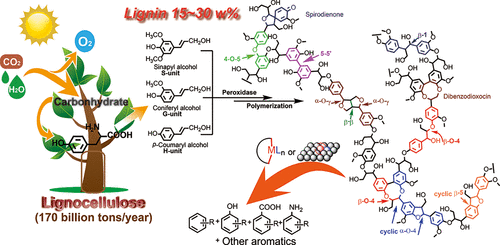
In recent decades, research on lignin depolymerization and its downstream product transformation has drawn an enormous amount of attention from academia to industry worldwide, aiming at harvesting aromatic compounds from this abundant and renewable biomass resource. Although the lignin conversion can be traced back to the 1930s and various noncatalytic and catalytic methods have been explored to depolymerize lignin via direct lignin conversion research or lignin models conversion studies, the complexity of the lignin structure, various linkages, the high stability of lignin bonds, and the diverse fragments condensation process make lignin depolymerization to monomers a highly challenging task. For the potential practical utilization of lignin, compared with lignin conversion to liquid fuel with extra H2 consumption, maintaining the aromatic structure and preparing high-value aromatic chemicals from renewable lignin is more profitable. Therefore, lignin depolymerization to easy-to-handle aromatic monomers with acceptable conversion and selectivity is of great importance. In this article, we present our recent studies on lignin's catalytic conversion to aromatic chemicals. First, we introduce our research on protolignin depolymerization via a fragmentation-hydrogenolysis process in alcohol solvents. Then, focusing on the catalytic cleavage of lignin C-C and C-O bonds, we shed light on a recapitulative adjacent functional group modification (AFGM) strategy for the conversion of lignin models. AFGM strategy begins with the adjacent functional group modification of the target C-C or C-O bond to directly decrease the bond dissociation enthalpy (BDE) of targeted bonds or generate new substrate sites to introduce the cleavage reagent for further conversion. Subsequently, on the basis of these two concepts from AFGM, we summarize our strategies on lignin depolymerization, which highlight the effects of lignin structure, catalyst character, and reaction conditions on the efficiency of strategies. In short, the key point for lignin depolymerization to aromatics is promoting the lignin conversion and restraining the condensation. Compared with the complex research on direct lignin conversion, this bottom-up research approach, beginning with lignin model research, can make the conversion mechanism study clear and provide potential methods for the protolignin/technical lignin conversion. In addition, one of our perspectives for lignin utilization is that the products from lignin conversion can be used as monomers for artificial polymerization, such as the simple phenol (PhOH) and other potential acid compounds, or that lignin derivative molecules can be used to synthesize high-value synthetic building blocks.
中文翻译:

催化木质素解聚为芳烃。
近几十年来,有关木质素解聚及其下游产物转化的研究已引起学术界到全世界工业的广泛关注,旨在从这种丰富的可再生生物质资源中收获芳香族化合物。尽管木质素转化可以追溯到1930年代,并且已经通过直接木质素转化研究或木质素模型转化研究,木质素结构的复杂性,各种连接,木质素键的高稳定性,探索了各种非催化和催化方法来解聚木质素。 ,以及各种片段的缩合过程使木质素解聚为单体是一项极富挑战性的任务。为了将木质素潜在地实际利用,与将木质素转化为具有额外H2消耗的液体燃料相比,保持芳族结构并从可再生木质素制备高价值的芳族化学品将更有利可图。因此,木质素解聚为易于处理的具有可接受的转化率和选择性的芳族单体非常重要。在本文中,我们介绍了有关木质素催化转化为芳族化学品的最新研究。首先,我们介绍了在醇类溶剂中通过裂解-氢解过程对原木脂素解聚的研究。然后,着重于木质素CC和CO键的催化裂解,我们阐明了用于木质素模型转化的概括性相邻官能团修饰(AFGM)策略。AFGM策略始于目标CC或CO键的相邻官能团修饰,以直接降低目标键的键解离焓(BDE)或生成新的底物位点以引入裂解试剂以进一步转化。随后,基于AFGM的这两个概念,我们总结了木质素解聚的策略,重点介绍了木质素结构,催化剂特性和反应条件对策略效率的影响。简而言之,木质素解聚为芳族化合物的关键是促进木质素转化并抑制缩合。与直接木质素转化的复杂研究相比,这种自下而上的研究方法始于木质素模型研究,可以使转化机理研究更加清晰,并为原木质素/技术木质素转化提供潜在的方法。此外,我们对木质素利用的观点之一是,木质素转化产生的产物可以用作人工聚合的单体,例如简单的酚(PhOH)和其他潜在的酸化合物,或者木质素衍生物分子可以用于合成高价值的合成积木。
更新日期:2020-01-31
中文翻译:

催化木质素解聚为芳烃。
近几十年来,有关木质素解聚及其下游产物转化的研究已引起学术界到全世界工业的广泛关注,旨在从这种丰富的可再生生物质资源中收获芳香族化合物。尽管木质素转化可以追溯到1930年代,并且已经通过直接木质素转化研究或木质素模型转化研究,木质素结构的复杂性,各种连接,木质素键的高稳定性,探索了各种非催化和催化方法来解聚木质素。 ,以及各种片段的缩合过程使木质素解聚为单体是一项极富挑战性的任务。为了将木质素潜在地实际利用,与将木质素转化为具有额外H2消耗的液体燃料相比,保持芳族结构并从可再生木质素制备高价值的芳族化学品将更有利可图。因此,木质素解聚为易于处理的具有可接受的转化率和选择性的芳族单体非常重要。在本文中,我们介绍了有关木质素催化转化为芳族化学品的最新研究。首先,我们介绍了在醇类溶剂中通过裂解-氢解过程对原木脂素解聚的研究。然后,着重于木质素CC和CO键的催化裂解,我们阐明了用于木质素模型转化的概括性相邻官能团修饰(AFGM)策略。AFGM策略始于目标CC或CO键的相邻官能团修饰,以直接降低目标键的键解离焓(BDE)或生成新的底物位点以引入裂解试剂以进一步转化。随后,基于AFGM的这两个概念,我们总结了木质素解聚的策略,重点介绍了木质素结构,催化剂特性和反应条件对策略效率的影响。简而言之,木质素解聚为芳族化合物的关键是促进木质素转化并抑制缩合。与直接木质素转化的复杂研究相比,这种自下而上的研究方法始于木质素模型研究,可以使转化机理研究更加清晰,并为原木质素/技术木质素转化提供潜在的方法。此外,我们对木质素利用的观点之一是,木质素转化产生的产物可以用作人工聚合的单体,例如简单的酚(PhOH)和其他潜在的酸化合物,或者木质素衍生物分子可以用于合成高价值的合成积木。











































 京公网安备 11010802027423号
京公网安备 11010802027423号