当前位置:
X-MOL 学术
›
npj Vaccines
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial.
npj Vaccines ( IF 6.9 ) Pub Date : 2020-01-31 , DOI: 10.1038/s41541-020-0160-2 Antje Blank 1 , Kristin Fürle 2 , Anja Jäschke 2 , Gerd Mikus 1 , Monika Lehmann 3 , Johannes Hüsing 3 , Kirsten Heiss 4 , Thomas Giese 5 , Darrick Carter 6 , Ernst Böhnlein 7 , Michael Lanzer 2 , Walter E Haefeli 1 , Hermann Bujard 7, 8
npj Vaccines ( IF 6.9 ) Pub Date : 2020-01-31 , DOI: 10.1038/s41541-020-0160-2 Antje Blank 1 , Kristin Fürle 2 , Anja Jäschke 2 , Gerd Mikus 1 , Monika Lehmann 3 , Johannes Hüsing 3 , Kirsten Heiss 4 , Thomas Giese 5 , Darrick Carter 6 , Ernst Böhnlein 7 , Michael Lanzer 2 , Walter E Haefeli 1 , Hermann Bujard 7, 8
Affiliation
|
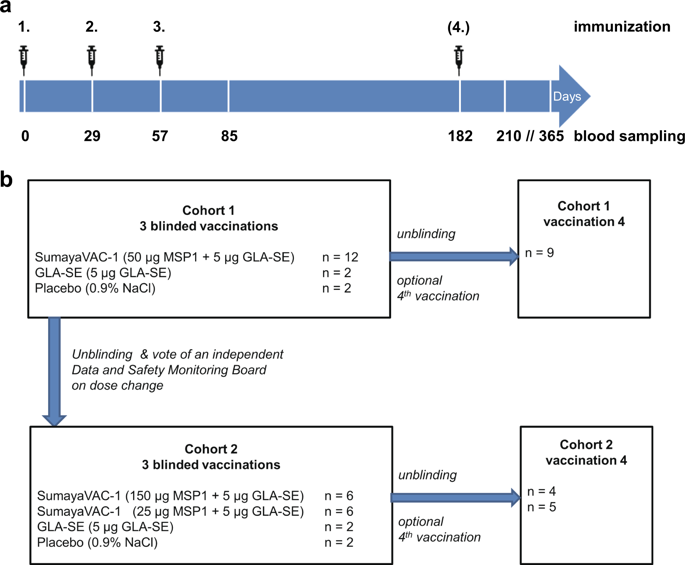
A vaccine remains a priority in the global fight against malaria. Here, we report on a single-center, randomized, double-blind, placebo and adjuvant-controlled, dose escalation phase 1a safety and immunogenicity clinical trial of full-length Plasmodium falciparum merozoite surface protein 1 (MSP1) in combination with GLA-SE adjuvant. Thirty-two healthy volunteers were vaccinated at least three times with MSP1 plus adjuvant, adjuvant alone, or placebo (24:4:4) to evaluate the safety and immunogenicity. MSP1 was safe, well tolerated and immunogenic, with all vaccinees sero-converting independent of the dose. The MSP1-specific IgG and IgM titers persisted above levels found in malaria semi-immune humans for at least 6 months after the last immunization. The antibodies were variant- and strain-transcending and stimulated respiratory activity in granulocytes. Furthermore, full-length MSP1 induced memory T-cells. Our findings encourage challenge studies as the next step to evaluate the efficacy of full-length MSP1 as a vaccine candidate against falciparum malaria (EudraCT 2016-002463-33).
中文翻译:

在一项随机首次人体试验中,使用全长恶性疟原虫裂殖子表面蛋白 1 进行免疫是安全的,并且可引发功能性亲细胞抗体。
疫苗仍然是全球抗击疟疾的首要任务。在这里,我们报告了一项单中心、随机、双盲、安慰剂和佐剂对照、剂量递增的 1a 期安全性和免疫原性临床试验,该试验涉及全长恶性疟原虫裂殖子表面蛋白 1 (MSP1) 与 GLA-SE 的组合佐剂。 32 名健康志愿者分别接种 MSP1 加佐剂、单独佐剂或安慰剂 (24:4:4) 至少 3 次,以评估安全性和免疫原性。 MSP1 安全、耐受性良好且具有免疫原性,所有疫苗接种者的血清转化与剂量无关。在最后一次免疫后至少 6 个月内,MSP1 特异性 IgG 和 IgM 滴度持续高于疟疾半免疫人类的水平。这些抗体能够超越变异和菌株,并刺激粒细胞的呼吸活动。此外,全长 MSP1 诱导记忆 T 细胞。我们的研究结果鼓励下一步开展挑战研究,以评估全长 MSP1 作为恶性疟疾候选疫苗的功效 (EudraCT 2016-002463-33)。
更新日期:2020-01-31
中文翻译:

在一项随机首次人体试验中,使用全长恶性疟原虫裂殖子表面蛋白 1 进行免疫是安全的,并且可引发功能性亲细胞抗体。
疫苗仍然是全球抗击疟疾的首要任务。在这里,我们报告了一项单中心、随机、双盲、安慰剂和佐剂对照、剂量递增的 1a 期安全性和免疫原性临床试验,该试验涉及全长恶性疟原虫裂殖子表面蛋白 1 (MSP1) 与 GLA-SE 的组合佐剂。 32 名健康志愿者分别接种 MSP1 加佐剂、单独佐剂或安慰剂 (24:4:4) 至少 3 次,以评估安全性和免疫原性。 MSP1 安全、耐受性良好且具有免疫原性,所有疫苗接种者的血清转化与剂量无关。在最后一次免疫后至少 6 个月内,MSP1 特异性 IgG 和 IgM 滴度持续高于疟疾半免疫人类的水平。这些抗体能够超越变异和菌株,并刺激粒细胞的呼吸活动。此外,全长 MSP1 诱导记忆 T 细胞。我们的研究结果鼓励下一步开展挑战研究,以评估全长 MSP1 作为恶性疟疾候选疫苗的功效 (EudraCT 2016-002463-33)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号