当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
3′-(4-(Benzyloxy)phenyl)-1′-phenyl-5-(heteroaryl/aryl)-3,4-dihydro-1′H ,2H -[3,4′-bipyrazole]-2-carboxamides as EGFR kinase inhibitors: Synthesis, anticancer evaluation, and molecular docking studies
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2020-04-01 , DOI: 10.1002/ardp.201900262 Farah Nawaz 1 , Ozair Alam 1 , Ahmad Perwez 2 , Moshahid A Rizvi 2 , Mohd J Naim 1 , Nadeem Siddiqui 1 , Faheem H Pottoo 3 , Mukund Jha 1
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2020-04-01 , DOI: 10.1002/ardp.201900262 Farah Nawaz 1 , Ozair Alam 1 , Ahmad Perwez 2 , Moshahid A Rizvi 2 , Mohd J Naim 1 , Nadeem Siddiqui 1 , Faheem H Pottoo 3 , Mukund Jha 1
Affiliation
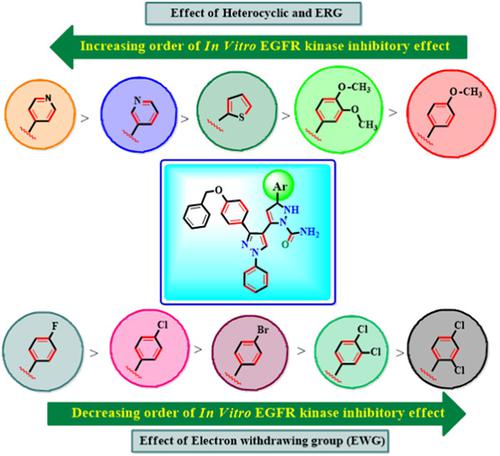
|
Pyrazoline‐linked carboxamide derivatives were designed, synthesized, and evaluated for potential epidermal growth factor receptor (EGFR) kinase inhibition, anticancer activity, and apoptotic and cardiomyopathy toxicity. Compounds 6m and 6n inhibit EGFR kinase at a concentration of 6.5 ± 2.91 and 3.65 ± 0.54 µM, respectively. Some of these compounds showed effects on proliferation, which were also then evaluated against four different human cancer cell lines, that is, MCF‐7 (breast cancer), A549 (non‐small‐cell lung tumor), HCT‐116 (colon cancer), and SiHa cells (cancerous tissues of the cervix uteri). The results showed that certain synthetic compounds showed significant inhibitor activity; compounds 6m and 6n were more cytotoxic than doxorubicin against A549 cancer cells, with IC50 values of 10.3 ± 1.07 and 4.6 ± 0.57 µM, respectively. Additionally, compounds 6m and 6n induced apoptosis in A549 cancer cells, as evidenced by 4′,6‐diamidino‐2‐phenylindole (DAPI) staining and phase‐contrast microscopy. Potency to induce apoptosis by compound 6n was further confirmed by fluorescence‐activated cell sorting using Annexin V‐FITC and propidium iodide labeling. Compound 6n showed normal cardiomyocytes with no marked sign of pyknotic nuclei in cardiomyopathy and also normal histological appearance of the renal cortex when compared with that of control. Results of molecular docking studies suggested that compounds 6m and 6n can bind to the hinge region of the adenosine triphosphate‐binding site of EGFR kinase, like the standard drug erlotinib. Therefore, the present study suggests that compounds 6m and 6n have potent in vitro antitumor activities against the human non‐small‐cell lung tumor cell line A549, which can be further explored in other cancer cell lines and in animal studies.
中文翻译:

3'-(4-(Benzyloxy)phenyl)-1'-phenyl-5-(heteroaryl/aryl)-3,4-dihydro-1'H,2H -[3,4'-bipyrazole]-2-carboxamides EGFR激酶抑制剂:合成、抗癌评估和分子对接研究
设计、合成了吡唑啉连接的甲酰胺衍生物,并评估了其潜在的表皮生长因子受体 (EGFR) 激酶抑制、抗癌活性以及细胞凋亡和心肌病毒性。化合物 6m 和 6n 分别以 6.5 ± 2.91 和 3.65 ± 0.54 µM 的浓度抑制 EGFR 激酶。其中一些化合物显示出对增殖的影响,然后还针对四种不同的人类癌细胞系进行了评估,即 MCF-7(乳腺癌)、A549(非小细胞肺癌)、HCT-116(结肠癌) )和 SiHa 细胞(子宫颈癌组织)。结果表明,某些合成化合物表现出显着的抑制剂活性;化合物 6m 和 6n 对 A549 癌细胞的细胞毒性比阿霉素更强,IC50 值分别为 10.3 ± 1.07 和 4.6 ± 0.57 µM。此外,化合物 6m 和 6n 诱导 A549 癌细胞凋亡,如 4',6-二脒基-2-苯基吲哚 (DAPI) 染色和相差显微镜所证明。使用膜联蛋白 V-FITC 和碘化丙啶标记的荧光激活细胞分选进一步证实了化合物 6n 诱导细胞凋亡的效力。与对照相比,化合物6n显示出正常的心肌细胞,在心肌病中没有明显的固缩核迹象,并且肾皮质的组织学外观也正常。分子对接研究结果表明,化合物 6m 和 6n 可以与标准药物厄洛替尼一样与 EGFR 激酶三磷酸腺苷结合位点的铰链区结合。所以,
更新日期:2020-04-01
中文翻译:

3'-(4-(Benzyloxy)phenyl)-1'-phenyl-5-(heteroaryl/aryl)-3,4-dihydro-1'H,2H -[3,4'-bipyrazole]-2-carboxamides EGFR激酶抑制剂:合成、抗癌评估和分子对接研究
设计、合成了吡唑啉连接的甲酰胺衍生物,并评估了其潜在的表皮生长因子受体 (EGFR) 激酶抑制、抗癌活性以及细胞凋亡和心肌病毒性。化合物 6m 和 6n 分别以 6.5 ± 2.91 和 3.65 ± 0.54 µM 的浓度抑制 EGFR 激酶。其中一些化合物显示出对增殖的影响,然后还针对四种不同的人类癌细胞系进行了评估,即 MCF-7(乳腺癌)、A549(非小细胞肺癌)、HCT-116(结肠癌) )和 SiHa 细胞(子宫颈癌组织)。结果表明,某些合成化合物表现出显着的抑制剂活性;化合物 6m 和 6n 对 A549 癌细胞的细胞毒性比阿霉素更强,IC50 值分别为 10.3 ± 1.07 和 4.6 ± 0.57 µM。此外,化合物 6m 和 6n 诱导 A549 癌细胞凋亡,如 4',6-二脒基-2-苯基吲哚 (DAPI) 染色和相差显微镜所证明。使用膜联蛋白 V-FITC 和碘化丙啶标记的荧光激活细胞分选进一步证实了化合物 6n 诱导细胞凋亡的效力。与对照相比,化合物6n显示出正常的心肌细胞,在心肌病中没有明显的固缩核迹象,并且肾皮质的组织学外观也正常。分子对接研究结果表明,化合物 6m 和 6n 可以与标准药物厄洛替尼一样与 EGFR 激酶三磷酸腺苷结合位点的铰链区结合。所以,



























 京公网安备 11010802027423号
京公网安备 11010802027423号