当前位置:
X-MOL 学术
›
J. Steroid Biochem. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CYP17A1 exhibits 17αhydroxylase/17,20-lyase activity towards 11β-hydroxyprogesterone and 11-ketoprogesterone metabolites in the C11-oxy backdoor pathway.
The Journal of Steroid Biochemistry and Molecular Biology ( IF 2.7 ) Pub Date : 2020-01-30 , DOI: 10.1016/j.jsbmb.2020.105614 Desmaré van Rooyen 1 , Rahul Yadav 2 , Emily E Scott 3 , Amanda C Swart 1
The Journal of Steroid Biochemistry and Molecular Biology ( IF 2.7 ) Pub Date : 2020-01-30 , DOI: 10.1016/j.jsbmb.2020.105614 Desmaré van Rooyen 1 , Rahul Yadav 2 , Emily E Scott 3 , Amanda C Swart 1
Affiliation
|
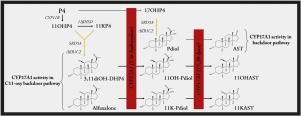
Cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1) plays a pivotal role in the regulation of adrenal and gonadal steroid hormone biosynthesis. More recent studies highlighted the enzyme's role in the backdoor pathway leading to androgen production. Increased CYP17A1 activity in endocrine disorders and diseases are associated with elevated C21 and C19 steroids which include 17α-hydroxyprogesterone and androgens, as well as C11-oxy C21 and C11-oxy C19 steroids. We previously reported that 11β-hydroxyprogesterone (11OHP4), 21-deoxycortisol (21dF) and their keto derivatives are converted by 5α-reductases and hydroxysteroid dehydrogenases yielding C19 steroids in the backdoor pathway. In this study the 17α-hydroxylase and 17,20-lyase activity of CYP17A1 towards the unconventional C11-oxy C21 steroid substrates and their 5α- and 3α,5α-reduced metabolites was investigated in transfected HEK-293 cells. CYP17A1 catalysed the 17α-hydroxylation of 11OHP4 to 21dF and 11-ketoprogesterone (11KP4) to 21-deoxycortisone (21dE) with negligible hydroxylation of their 5α-reduced metabolites while no lyase activity was detected. The 3α,5α-reduced C11-oxy C21 steroids-5α-pregnan-3α,11β-diol-20-one (3,11diOH-DHP4) and 5α-pregnan-3α-ol-11,20-dione (alfaxalone) were rapidly hydroxylated to 5α-pregnan-3α,11β,17α-triol-20-one (11OH-Pdiol) and 5α-pregnan-3α,17α-diol-11,20-dione (11K-Pdiol), with the lyase activity subsequently catalysing to conversion to the C11-oxy C19 steroids, 11β-hydroxyandrosterone and 11-ketoandrosterone, respectively. Docking of 11OHP4, 11KP4 and the 5α-reduced metabolites, 5α-pregnan-11β-ol-3,20-dione (11OH-DHP4) and 5α-pregnan-3,11,20-trione (11K-DHP4) with human CYP17A1 showed minimal changes in the orientation of these C11-oxy C21 steroids in the active pocket when compared with the binding of progesterone suggesting the 17,20-lyase is impaired by the C11-hydroxyl and keto moieties. The structurally similar 3,11diOH-DHP4 and alfaxalone showed a greater distance between C17 and the heme group compared to the natural substrate, 17α-hydroxypregnenolone potentially allowing more orientational freedom and facilitating the conversion of the C11-oxy C21 to C11-oxy C19 steroids. In summary, our in vitro assays showed that while CYP17A1 readily hydroxylated 11OHP4 and 11KP4, the enzyme was unable to catalyse the 17,20-lyase reaction of these C11-oxy C21 steroid products. Although CYP17A1 exhibited no catalytic activity towards the 5α-reduced intermediates, once the C4-C5 double bond and the keto group at C3 were reduced, both the hydroxylation and lyase reactions proceeded efficiently. These findings show that the C11-oxy C21 steroids could potentially contribute to the androgen pool in tissue expressing steroidogenic enzymes in the backdoor pathway.
中文翻译:

CYP17A1对C11-oxy后门途径中的11β-羟孕酮和11-酮戊二酮代谢物具有17α羟化酶/ 17,20-裂合酶活性。
细胞色素P45017α-羟化酶/ 17,20-裂解酶(CYP17A1)在调节肾上腺和性腺类固醇激素的生物合成中起关键作用。最近的研究强调了该酶在导致雄激素生成的后门途径中的作用。内分泌失调和疾病中CYP17A1活性的增加与C21和C19类固醇升高有关,其中包括17α-羟基孕酮和雄激素以及C11-氧基C21和C11-氧基C19类固醇。我们先前曾报道11β-羟基孕酮(11OHP4),21-脱氧皮质醇(21dF)及其酮衍生物被5α-还原酶和羟基类固醇脱氢酶转化,从而在后门途径中产生C19类固醇。在这项研究中,CYP17A1对非常规的C11-氧基C21类固醇底物及其5α-和3α的17α-羟化酶和17,20-裂合酶活性,在转染的HEK-293细胞中研究了5α还原的代谢产物。CYP17A1催化11OHP4的17α-羟基化为21dF和11-酮戊二酮(11KP4)变为21-脱氧可的松(21dE),而其5α还原的代谢物的羟基化作用可忽略不计。3α,5α还原的C11-氧基C21类固醇5α-pregnan-3α,11β-二醇-20-一(3,11diOH-DHP4)和5α-pregnan-3α-ol-11,20-二酮(阿法沙酮)迅速羟基化为5α-pregnan-3α,11β,17α-triol-20-one(11OH-Pdiol)和5α-pregnan-3α,17α-diol-11,20-dione(11K-Pdiol),随后具有裂解酶活性分别催化转化为C11-氧基C19类固醇11β-羟基雄甾酮和11-酮雄甾酮。对接11OHP4、11KP4和5α还原的代谢物5α-pregnan-11β-ol-3,20-dione(11OH-DHP4)和5α-pregnan-3,11,与孕酮的结合相比,具有人CYP17A1的20-三酮(11K-DHP4)在活性口袋中的这些C11-氧基C21类固醇的取向变化很小,这表明17,20-裂解酶受C11-羟基和酮基部分。与天然底物相比,结构相似的3,11diOH-DHP4和阿法沙酮显示C17和血红素基团之间的距离更大,17α-羟基孕烯醇酮可能允许更多的取向自由并促进C11-氧基C21向C11-氧基C19类固醇的转化。总之,我们的体外试验表明,虽然CYP17A1容易将11OHP4和11KP4羟基化,但该酶无法催化这些C11-氧基C21类固醇产物的17,20-裂解酶反应。尽管CYP17A1对5α还原的中间体没有催化活性,一旦C4-C5双键和C3的酮基被还原,羟化反应和裂解酶反应均有效进行。这些发现表明,C11-氧基C21类固醇可能会在后门途径中表达类固醇生成酶的组织中促进雄激素库的形成。
更新日期:2020-01-31
中文翻译:

CYP17A1对C11-oxy后门途径中的11β-羟孕酮和11-酮戊二酮代谢物具有17α羟化酶/ 17,20-裂合酶活性。
细胞色素P45017α-羟化酶/ 17,20-裂解酶(CYP17A1)在调节肾上腺和性腺类固醇激素的生物合成中起关键作用。最近的研究强调了该酶在导致雄激素生成的后门途径中的作用。内分泌失调和疾病中CYP17A1活性的增加与C21和C19类固醇升高有关,其中包括17α-羟基孕酮和雄激素以及C11-氧基C21和C11-氧基C19类固醇。我们先前曾报道11β-羟基孕酮(11OHP4),21-脱氧皮质醇(21dF)及其酮衍生物被5α-还原酶和羟基类固醇脱氢酶转化,从而在后门途径中产生C19类固醇。在这项研究中,CYP17A1对非常规的C11-氧基C21类固醇底物及其5α-和3α的17α-羟化酶和17,20-裂合酶活性,在转染的HEK-293细胞中研究了5α还原的代谢产物。CYP17A1催化11OHP4的17α-羟基化为21dF和11-酮戊二酮(11KP4)变为21-脱氧可的松(21dE),而其5α还原的代谢物的羟基化作用可忽略不计。3α,5α还原的C11-氧基C21类固醇5α-pregnan-3α,11β-二醇-20-一(3,11diOH-DHP4)和5α-pregnan-3α-ol-11,20-二酮(阿法沙酮)迅速羟基化为5α-pregnan-3α,11β,17α-triol-20-one(11OH-Pdiol)和5α-pregnan-3α,17α-diol-11,20-dione(11K-Pdiol),随后具有裂解酶活性分别催化转化为C11-氧基C19类固醇11β-羟基雄甾酮和11-酮雄甾酮。对接11OHP4、11KP4和5α还原的代谢物5α-pregnan-11β-ol-3,20-dione(11OH-DHP4)和5α-pregnan-3,11,与孕酮的结合相比,具有人CYP17A1的20-三酮(11K-DHP4)在活性口袋中的这些C11-氧基C21类固醇的取向变化很小,这表明17,20-裂解酶受C11-羟基和酮基部分。与天然底物相比,结构相似的3,11diOH-DHP4和阿法沙酮显示C17和血红素基团之间的距离更大,17α-羟基孕烯醇酮可能允许更多的取向自由并促进C11-氧基C21向C11-氧基C19类固醇的转化。总之,我们的体外试验表明,虽然CYP17A1容易将11OHP4和11KP4羟基化,但该酶无法催化这些C11-氧基C21类固醇产物的17,20-裂解酶反应。尽管CYP17A1对5α还原的中间体没有催化活性,一旦C4-C5双键和C3的酮基被还原,羟化反应和裂解酶反应均有效进行。这些发现表明,C11-氧基C21类固醇可能会在后门途径中表达类固醇生成酶的组织中促进雄激素库的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号