当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics of glycerol and diols valorisation via reactive systems of acetals synthesis
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fluid.2020.112503 Sergey P. Verevkin , Maria E. Konnova , Kseniya V. Zherikova , Andrey A. Pimerzin
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fluid.2020.112503 Sergey P. Verevkin , Maria E. Konnova , Kseniya V. Zherikova , Andrey A. Pimerzin
|
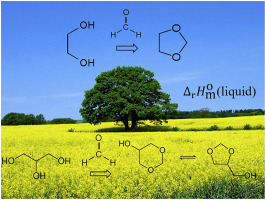
Abstract Glycerol and diols are valuable renewable sources for manufacturing of fuel additives via reactive systems of acetals synthesis. Knowledge of thermodynamic data for reactions participants involved in valorisation of glycerol and diols is required. The liquid phase standard molar enthalpy of formation of glycerol formal was measured by the high-precision combustion calorimetry. Standard molar enthalpies of vaporisation of glycerol formal, 1,3-dioxane, 1,4-dioxane, and dimethyl-1,3-dioxanes were derived from temperature dependences of vapour pressure measured with help of transpiration method. Temperature dependences of vapour pressures for acetals available in the literature have been collected and treated uniformly in order to derive recommended sets of vapour pressures and vaporisation enthalpies at the reference temperature 298.15 K. Enthalpies of vaporisation of these compounds were obtained by the transpiration method. Experimental results for glycerol formal were validated by the high-level G4 quantum-chemical method. Enthalpies of the acetalisation reactions of glycerol and diols were estimated and discussed.
中文翻译:

通过缩醛合成的反应系统使甘油和二醇增值的热力学
摘要 甘油和二醇是通过缩醛合成反应系统制造燃料添加剂的宝贵可再生资源。需要了解参与甘油和二醇价值化的反应参与者的热力学数据。通过高精度燃烧量热法测量了甘油缩甲醛的液相标准摩尔生成焓。甘油缩甲醛、1,3-二氧六环、1,4-二氧六环和二甲基-1,3-二氧六环的蒸发的标准摩尔焓源自借助蒸腾法测量的蒸气压的温度依赖性。收集了文献中可用的缩醛蒸气压的温度依赖性并对其进行了统一处理,以推导出参考温度 298.15 K 下推荐的蒸气压和蒸发焓组。这些化合物的蒸发焓是通过蒸腾法获得的。甘油缩甲醛的实验结果通过高级 G4 量子化学方法进行验证。估计和讨论了甘油和二醇的缩醛化反应的焓。
更新日期:2020-04-01
中文翻译:

通过缩醛合成的反应系统使甘油和二醇增值的热力学
摘要 甘油和二醇是通过缩醛合成反应系统制造燃料添加剂的宝贵可再生资源。需要了解参与甘油和二醇价值化的反应参与者的热力学数据。通过高精度燃烧量热法测量了甘油缩甲醛的液相标准摩尔生成焓。甘油缩甲醛、1,3-二氧六环、1,4-二氧六环和二甲基-1,3-二氧六环的蒸发的标准摩尔焓源自借助蒸腾法测量的蒸气压的温度依赖性。收集了文献中可用的缩醛蒸气压的温度依赖性并对其进行了统一处理,以推导出参考温度 298.15 K 下推荐的蒸气压和蒸发焓组。这些化合物的蒸发焓是通过蒸腾法获得的。甘油缩甲醛的实验结果通过高级 G4 量子化学方法进行验证。估计和讨论了甘油和二醇的缩醛化反应的焓。











































 京公网安备 11010802027423号
京公网安备 11010802027423号