当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the colonic disposition of celecoxib in humans.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.ejps.2020.105242 Glenn Lemmens 1 , Joachim Brouwers 1 , Jan Snoeys 2 , Patrick Augustijns 1 , Tim Vanuytsel 3
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.ejps.2020.105242 Glenn Lemmens 1 , Joachim Brouwers 1 , Jan Snoeys 2 , Patrick Augustijns 1 , Tim Vanuytsel 3
Affiliation
|
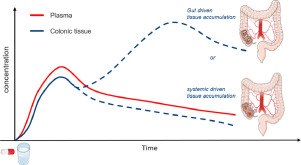
Although the effect of NSAIDs such as celecoxib on the progression of colorectal polyps has been established, it is currently unknown how sufficiently high concentrations of celecoxib are reached in colonic tissue. Indeed, the lipophilic and poorly soluble celecoxib is orally dosed as an immediate release capsule without any colon-targeting delivery strategy. In the present study, we aimed to distinguish between plasma and gut driven caecal tissue accumulation of celecoxib in healthy volunteers. After developing a protocol to reliably collect colonic biopsies and contents, the disposition of celecoxib was assessed in plasma, caecal tissue and caecal contents collected after intake of a celecoxib capsule (200 mg; Celebrex®) with 240 mL of tap water. During a first colonoscopy (1.0-2.5 h after drug intake), plasma concentrations of celecoxib and its carboxy metabolite were increasing, while caecal tissue concentrations were relatively low. As no celecoxib was present in caecal contents, tissue accumulation was clearly plasma driven. During a second colonoscopy (6.0-7.5 h after drug intake), tissue concentrations of the drug and its metabolite were substantially higher despite decreasing plasma concentrations. As a high amount of celecoxib was found in the caecal contents, the increased tissue accumulation most likely resulted from direct uptake of celecoxib from the gut. These data demonstrate that incomplete small intestinal absorption of the poorly soluble drug celecoxib enables gut driven drug accumulation in caecal tissue, which is, most likely, critical for the role of this NSAID in the prevention of colorectal cancer.
中文翻译:

深入了解塞来昔布在人体内的结肠处置。
尽管已经建立了诸如塞来昔布之类的非甾体抗炎药对大肠息肉进展的作用,但目前尚不清楚结肠组织中塞来昔布如何达到足够高的浓度。实际上,亲脂性和难溶性塞来昔布口服给药为速释胶囊,没有任何靶向结肠的递送策略。在本研究中,我们旨在区分健康志愿者中血浆和肠道驱动的塞来昔布盲肠组织积聚情况。在制定出可靠收集结肠活检标本和内容物的方案后,评估塞来昔布的血浆,盲肠组织和盲肠内容物的摄入情况,这些样品是在摄入240毫升自来水塞来昔布胶囊(200 mg;)后收集的。在第一次结肠镜检查期间(服药后1.0-2.5小时),塞来昔布及其羧基代谢产物的血浆浓度增加,而盲肠组织浓度相对较低。由于盲肠内容物中不存在塞来昔布,因此组织蓄积显然是血浆驱动的。在第二次结肠镜检查期间(服药后6.0-7.5小时),尽管血浆浓度降低,但药物及其代谢产物的组织浓度仍显着较高。由于在盲肠内容物中发现大量塞来昔布,因此组织蓄积的增加很可能是由于从肠道直接摄取塞来昔布引起的。这些数据表明,难溶性药物塞来昔布的小肠吸收不完全会导致肠道驱动的药物在盲肠组织中积聚,这对于该NSAID在预防结直肠癌中的作用至关重要。
更新日期:2020-01-31
中文翻译:

深入了解塞来昔布在人体内的结肠处置。
尽管已经建立了诸如塞来昔布之类的非甾体抗炎药对大肠息肉进展的作用,但目前尚不清楚结肠组织中塞来昔布如何达到足够高的浓度。实际上,亲脂性和难溶性塞来昔布口服给药为速释胶囊,没有任何靶向结肠的递送策略。在本研究中,我们旨在区分健康志愿者中血浆和肠道驱动的塞来昔布盲肠组织积聚情况。在制定出可靠收集结肠活检标本和内容物的方案后,评估塞来昔布的血浆,盲肠组织和盲肠内容物的摄入情况,这些样品是在摄入240毫升自来水塞来昔布胶囊(200 mg;)后收集的。在第一次结肠镜检查期间(服药后1.0-2.5小时),塞来昔布及其羧基代谢产物的血浆浓度增加,而盲肠组织浓度相对较低。由于盲肠内容物中不存在塞来昔布,因此组织蓄积显然是血浆驱动的。在第二次结肠镜检查期间(服药后6.0-7.5小时),尽管血浆浓度降低,但药物及其代谢产物的组织浓度仍显着较高。由于在盲肠内容物中发现大量塞来昔布,因此组织蓄积的增加很可能是由于从肠道直接摄取塞来昔布引起的。这些数据表明,难溶性药物塞来昔布的小肠吸收不完全会导致肠道驱动的药物在盲肠组织中积聚,这对于该NSAID在预防结直肠癌中的作用至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号